What’s Next in the Biotech Pipeline?
Investing in biotech stocks demands prudence combined with a sprinkling of optimism. This means taking in announcements from companies bragging about potential blockbuster drugs with a grain of salt.
After all, a single misstep towards gaining FDA approval could easily set back any progress, erase any hope of salvaging the discovery, and eventually, send their share prices spiraling down.
On the other hand, choosing a biotech stock that would deliver on its promise means reaping rich dividends in the future.
With all the developments in store though, it’s hard to see why 2020 can’t easily go down as The Year of Biotech. Here are some things that caught my eye.
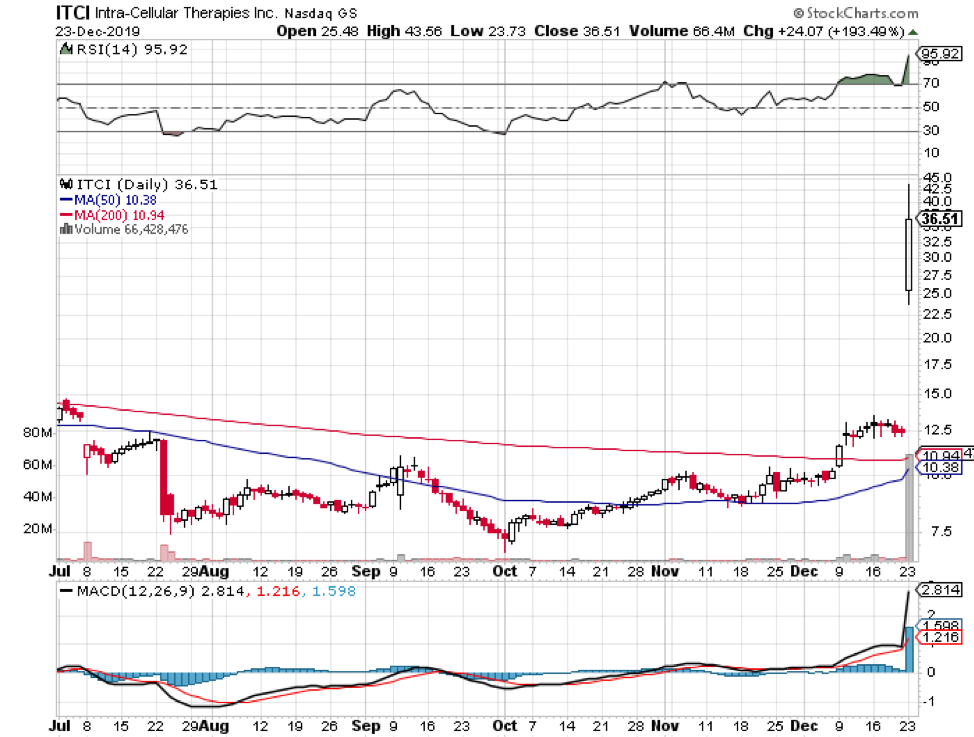
Christmas came early for Intra-Cellular Therapies (ITCI) as its long-awaited schizophrenia drug, Caplyta, received the green light from the FDA.
Although it has taken a few years for the biotech firm to announce the results of its schizophrenia trials, its investors are confident that Calpyta’s journey from this highly sought approval to marketing will be smooth sailing.
While the number of people suffering from schizophrenia and bipolar disorder is not as many as those facing major depressive disorder, treatments for the former conditions remain lacking. In fact, health specialists have been looking for more convenient options -- one that won’t hinder the daily lives of patients taking it.
This blockbuster drug, pegged as a safer and better alternative to Johnson and Johnson’s (JNJ) Risperidone, is expected to expand Lumateperone’s reach in the mental health market.
Despite earning an early victory, ITCI is already gearing up to tweak Calpyta’s indications and seek bipolar depression approvals as well. At the moment, this schizophrenia drug is estimated to cross $1 billion in sales following its 2020 launch in the market.
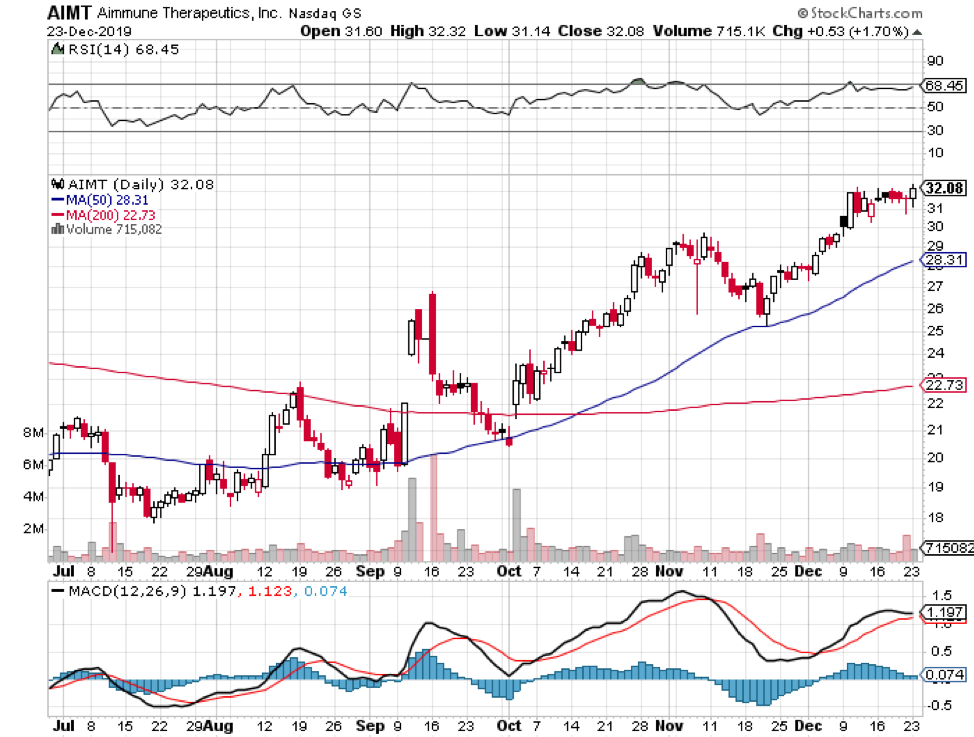
Meanwhile, there’s another big market drug that’s projected to make a major launch in 2020. Aimmune Therapeutics’ (AIMT) AR101. Otherwise known Palforzia, this will be the first-ever treatment for peanut allergies.
Although there’s no price tag released yet, a year’s supply of Palforzia is estimated to cost $4,200 per patient.
These pull-apart capsules, which are basically comprised of unmodified peanut flour plus a bunch of inactive ingredients, aim to provide medication for a food allergy that affects one in 13 children today.
The FDA is expected to release its decision on Palforzia sometime in January 2020, so it’ll definitely be a prosperous New Year for its investors.
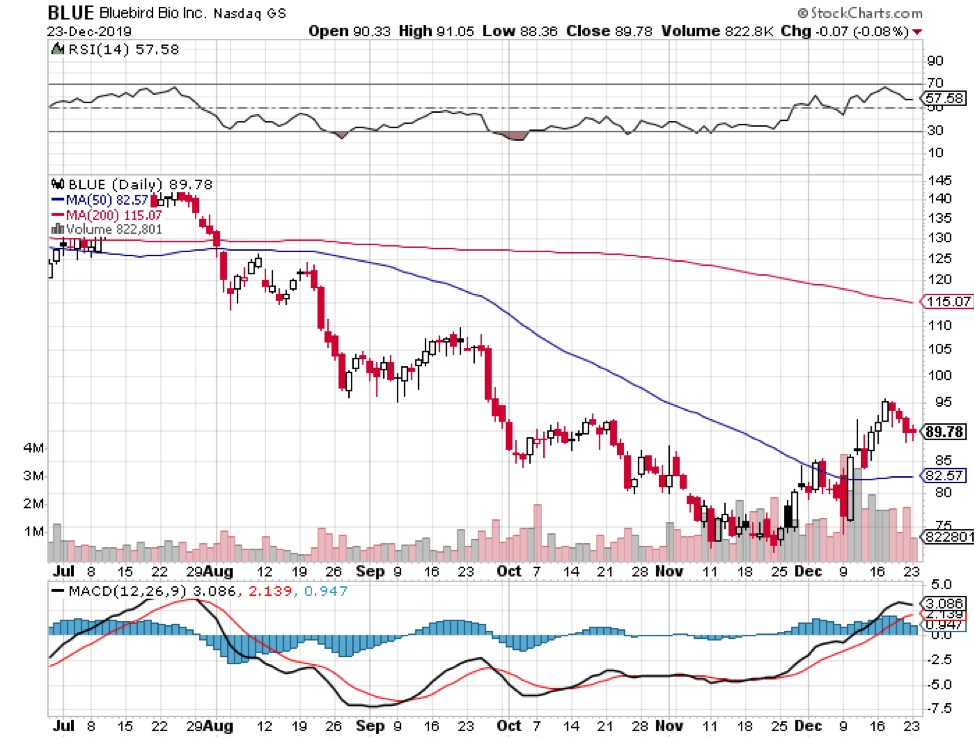
Another biotech company that’s set to make a splash in a lucrative market is Bluebird Bio (BIO).
At the moment, investors are chomping at the bit for good news concerning the company’s future crown jewel: genetic blood disease treatment Zynteglo.
In 2019, Zynteglo gained approval in the European market. Now, Bluebird is setting its sights to also conquer the US market as one in every 100,000 people is afflicted by this rare condition.
More than that, the only approved therapy for this genetic blood ailment is a blood transfusion done regularly.
Given the rarity of the disease and the efficacy of the treatment, Zynteglo’s price tag will obviously be on the high end.
This therapy is expected to cost roughly $1.8 million in total for every patient. To ease the burden though, Bluebird shared that it’s open to four- and five-year installment plans. That puts every gene therapy infusion at $355,000 per session.
Despite this massive expense, Bluebird actually believes that it’s selling its treatment at a discount of about 15% compared to the actual market value of $2.1 million.
This conviction comes from the fact that the company estimates adding 22 quality-adjusted life years to the lives of every successfully treated patient.
All three biotech stocks could easily skyrocket, especially Bluebird Bio. As for Aimmune Therapeutics, the company is currently financially healthy so it shouldn’t encounter any trouble meeting obligations. Meanwhile, ITCI has been gifting its investors with early Christmas presents since it first released promising results of its schizophrenia study.