Moat is a concept that Warren Buffett's followers are well-acquainted with.
In a nutshell, it describes a company’s capacity to keep its competitive edge over its rivals. For the Oracle of Omaha, the safest bets are businesses with large moats because it indicates a strong ability to ward off competitors.
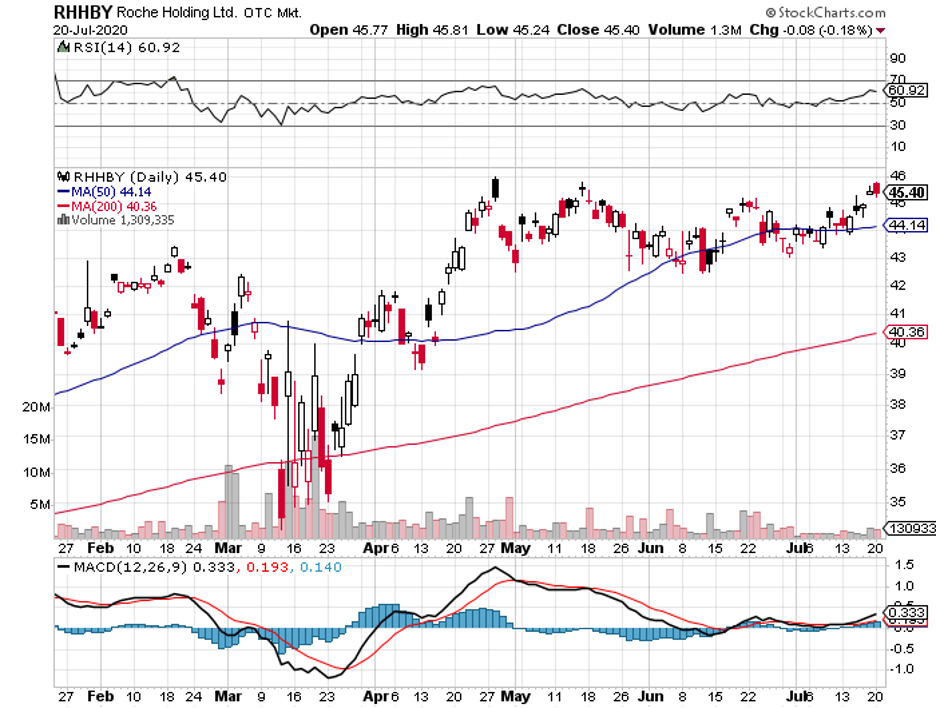
One company that has a particularly strong moat is Roche (RHHBY).
Roche has been at the forefront of the fight against the COVID-19 pandemic.
In mid-March, Roche became the first-ever commercial company to receive an FDA Emergency Use Authorization for its COVID-19 tests. What made this kit, called Cobas SARS-CoV-2 test, impressive is that the turnaround time of less than 4 hours was incredibly fast compared to others.
By April, Roche’s tests were already administered to roughly 4 million people, with some users paying as low as $5 for every test.
Following the success of its tests, Roche ventured into developing a COVID-19 cure.
While there’s still no conclusive data on its tests, Roche secured agreements with the European Commission to be one of the suppliers of the experimental COVID-19 drugs to any of the 27 EU members looking to buy for their constituents.
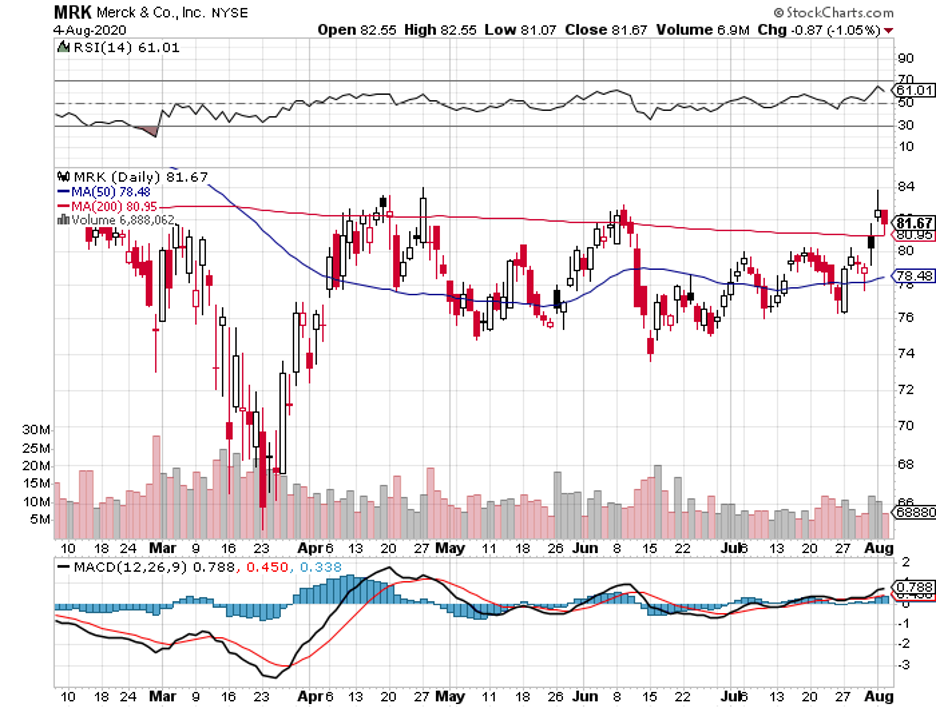
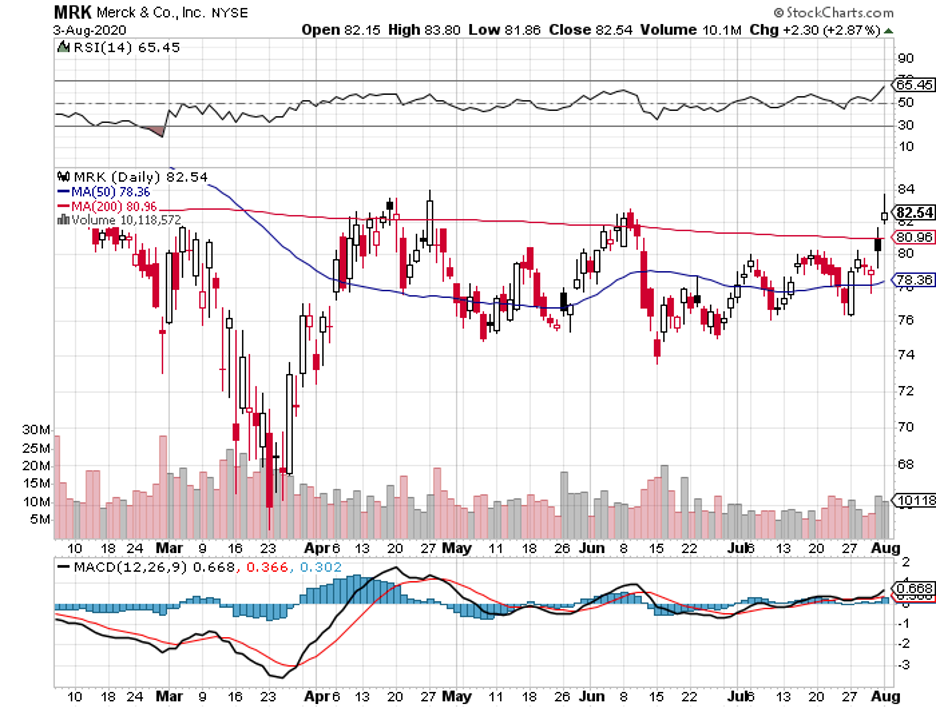
The deal involves Roche’s RoActemra. Meanwhile, the other supplier is Merck (MRK) with its Rebif.
Aside from that, Roche is also working alongside Gilead Sciences (GILD) in investigating whether Remdesivir could work better when combined with RoActemra.
The other drug undergoing similar compatibility tests with Remdesivir is Eli Lilly’s (LLY) Olumiant.
However, there remains a much bigger story for Roche outside its COVID-19 efforts.
Looking at the company’s first-quarter earnings report, Roche’s pharmaceutical arm generated over 80% of its total revenue for that period.
This is primarily thanks to its strong lineup of drugs, which recorded a 7% increase to reach roughly $13 billion in sales compared to the previous quarter. Overall, Roche recorded a 52.9% growth in its year-over-year quarterly earnings.
The key growth drivers of the company came from its oncology sector.
Leading the charge is bladder and urinary tract cancer treatment Tecentriq, followed by breast cancer drug Perjeta.
Roche’s efforts to expand the label of its blockbuster drug Tecentriq sets expectations for further growth as well.
To further boost its dominance in the oncology field, Roche recently signed an agreement with Blueprint Medicines (BPMC) to gain commercial rights to market thyroid and lung cancer treatment Pralsetinib outside the U.S., excluding Greater China.
This will allow Roche to directly compete with Eli Lilly’s newly gained blockbuster drug Rotovmo, which the company got from its $8 billion takeover of Loxo Oncology in 2019.
Apart from its oncology sector, Roche also saw promising results from other treatments like hemophilia medicine Hemlibra and multiple sclerosis treatment Ocrevus.
On top of Roche’s 37 approved treatments in the market today, the company is expected to submit regulatory findings for almost 20 products this year alone.
Meanwhile, Roche’s $4.3 billion acquisition of Spark Therapeutics in 2019 provided a much-need boost to the company’s gene therapy space.
Despite the uncertainties brought about by the pandemic, Roche’s shares still saw a 10.5% jump this year. In fact, the company increased its 2020 earnings estimate by 0.8% while it expects a 1.4% rise in 2021.
For context, Roche generated $61.5 billion in revenue in 2019 and raked in approximately $13.5 billion in profits. To date, the company pays its shareholders a dividend that yields close to 2.5%.
These reports highlight Roche’s financial stability and strength.
So far, Roche has been able to corner three of the major diseases today: cancer, hemophilia, and multiple sclerosis.
This makes the company one of the biggest names in the biotechnology and healthcare sector in terms of sales.
In fact, Roche is projected to be the No.1 in the field by 2026 with an annual revenue of $62 billion, achieving a compound rate of over 3.6% since its 2019 numbers.
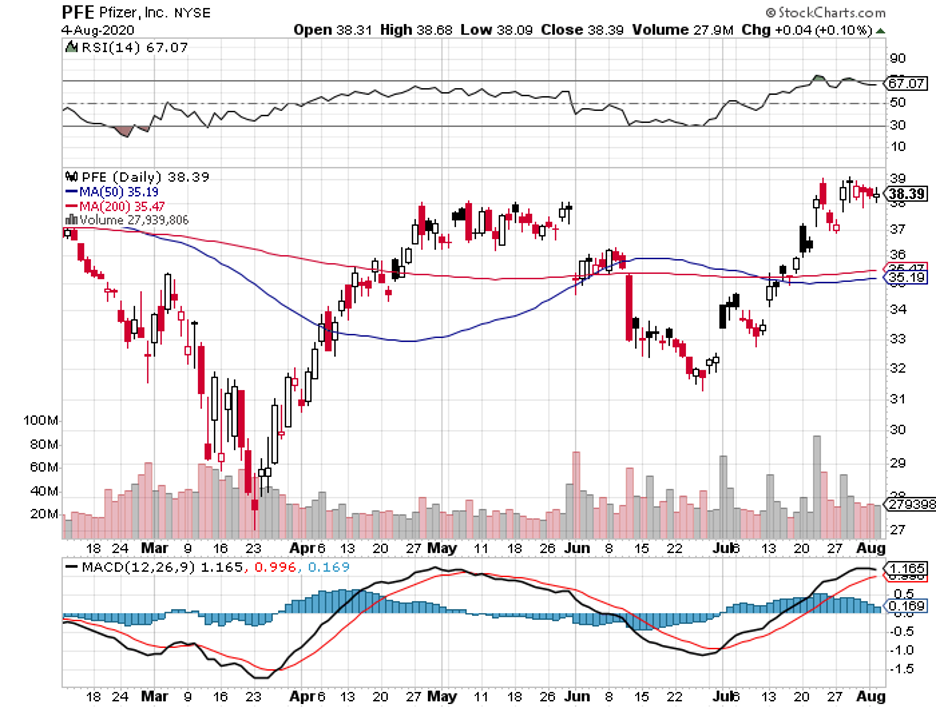
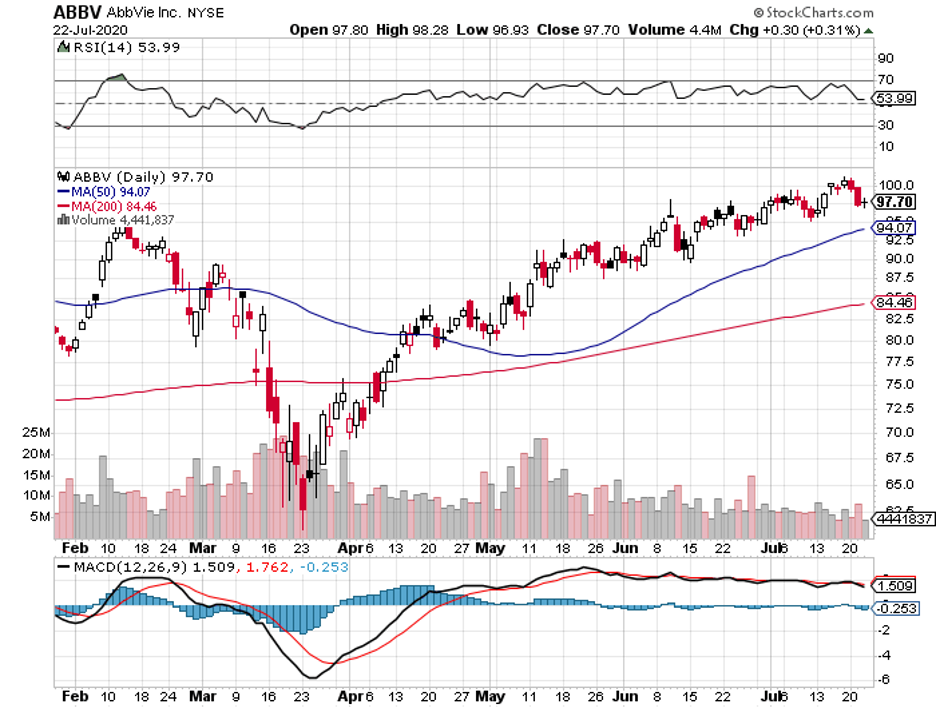
Pfizer (PFE) is expected to land second place, with sales estimated to reach over $56 billion. The rest of the list includes companies poised to record more than $50 billion in sales, namely, Johnson & Johnson (JNJ), AbbVie (ABBV), and Novartis (NVS).