In less than six months since the pandemic broke, the world has come up with 140 vaccine candidates in pre-clinical trial phase and 23 undergoing the clinical evaluation stage.
However, the race to produce a COVID-19 vaccine recently intensified after three of the leading laboratories shared promising progress from their early human trials.
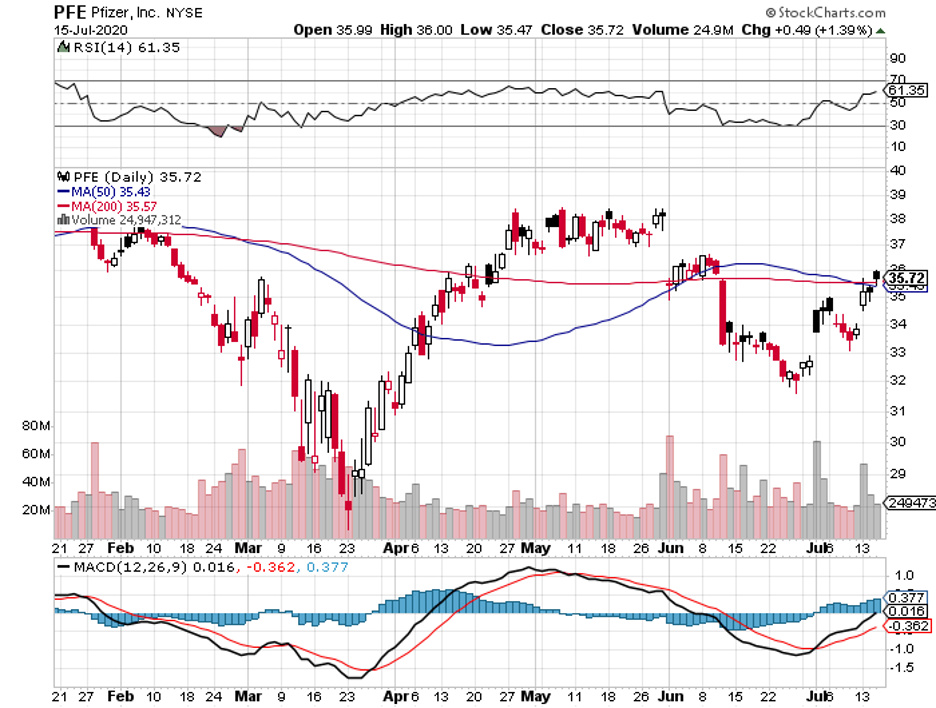
Among the frontrunners, AstraZeneca (AZN), Pfizer (PFE), and Moderna (MRNA) are dubbed as the “most ambitious” primarily due to their tight timelines for approval and their seemingly unreachable goals in terms of the total doses they could produce this year.
All the vaccine developers that released their results claimed that their candidates triggered strong immune responses with patients experiencing only minor side effects.
According to their data, their vaccines elicited similar responses as those observed from the individuals who recovered from COVID-19.
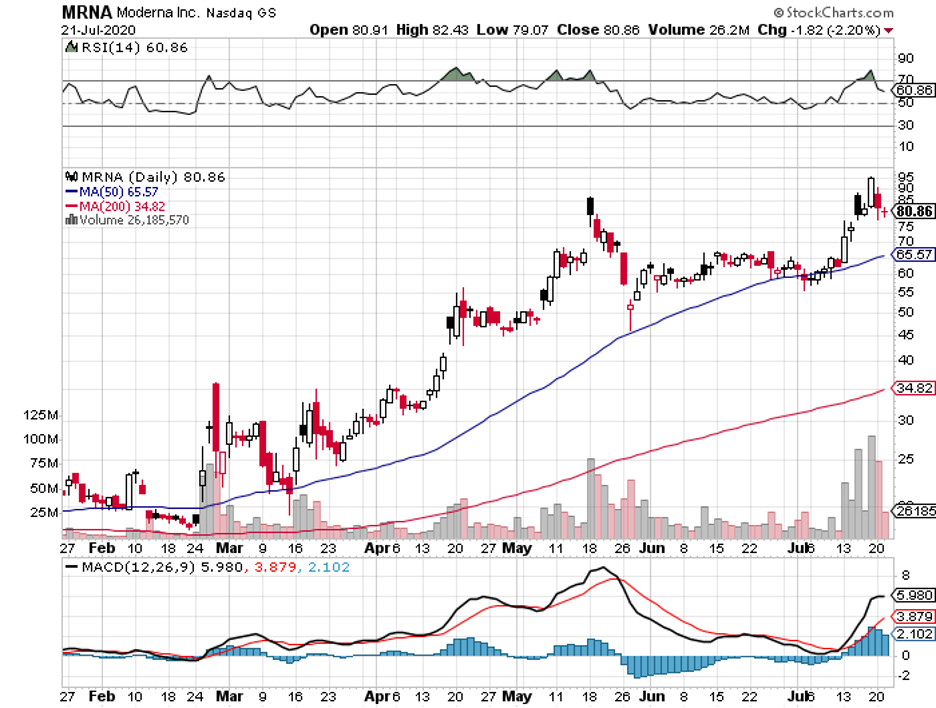
Moderna, which was the first developer to test its mRNA-1273 vaccine in humans, announced that Phase 3 of its human trials would start on July 27. This will involve 30,000 volunteers.
Moderna’s vaccine makes use of a genetic material, called mRNA, from the SARS-CoV-2 to trigger the body’s immune system to fight off the virus.
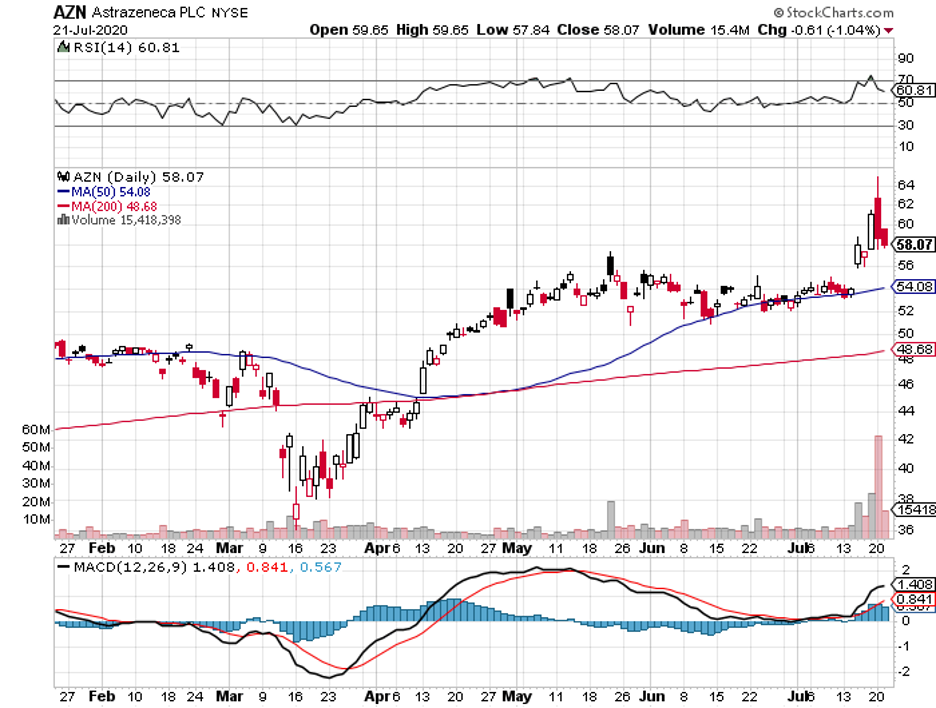
Meanwhile, AstraZeneca’s partnership with Oxford University produced what could be the most closely followed COVID-19 vaccine thus far. The candidate is tentatively known as AZD1222.
Even before the vaccine’s efficacy gets proven, the company already received a total order of 2 billion doses worldwide. Early estimates indicate that AstraZeneca can produce a million doses of its COVID-19 vaccine by September.
This partnership’s project is so sought after that rumors keep persisting that Russian spies are out to steal the research.
AstraZeneca’s approach is similar to the technique used by another vaccine maker outside the US, China’s own Cansino Biologics.
Both developers altered the genes of the adenovirus, which is another common virus, modifying it to harmlessly mimic the SARS-CoV-2. This will then induce an immune response from the body.
While Moderna was the first to enter human trials, AstraZeneca and Oxford’s candidate was the first to start the Phase 3 tests.
The reason that AZD1222 is gaining more traction than Moderna’s vaccine is because one dose of AstraZeneca’s candidate elicited an antibody response in more than 90% of the participants and a second dose managed to hit 100% -- the same level found among convalescent COVID-19 patients.
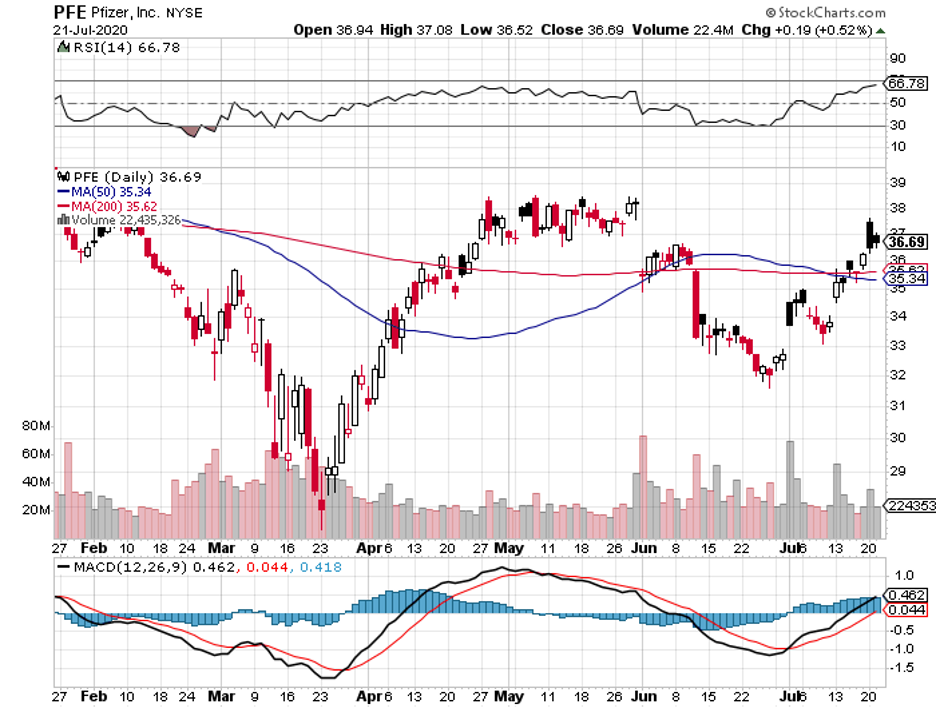
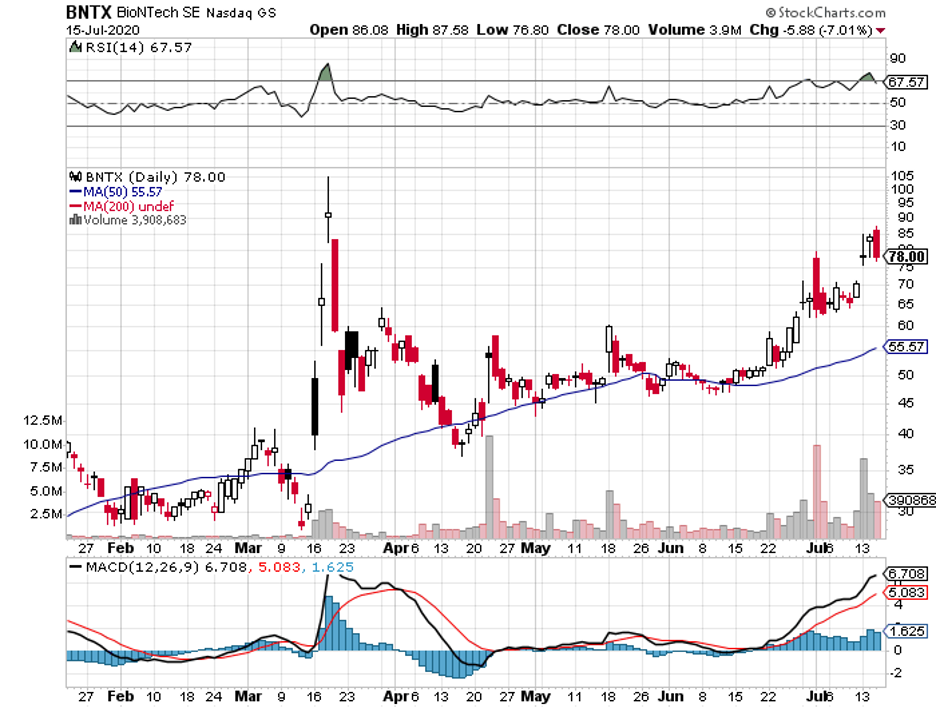
Among the three, though, Pfizer and BioNTech (BNTX) stand out because this is the only collaboration that refused government funding from the US.
This partnership uses the same approach as Moderna, with its early results also showing off promising immune responses from the participants.
Although the details have yet to be officially released, Pfizer landed its first government contract. The deal is with the UK and the New York-based biopharmaceutical company is expected to deliver 30 million vaccine doses between 2020 and 2021.
Aside from Moderna, AstraZeneca, and Pfizer, two more companies were included in Donald Trump’s Operation Warp Speed.
The fourth company is Johnson & Johnson (JNJ). The biotechnology and healthcare titan has yet to release results, with its first human trials scheduled this July and late-stage tests expected to start as soon as September. JNJ’s goal is to produce a vaccine by early 2021.
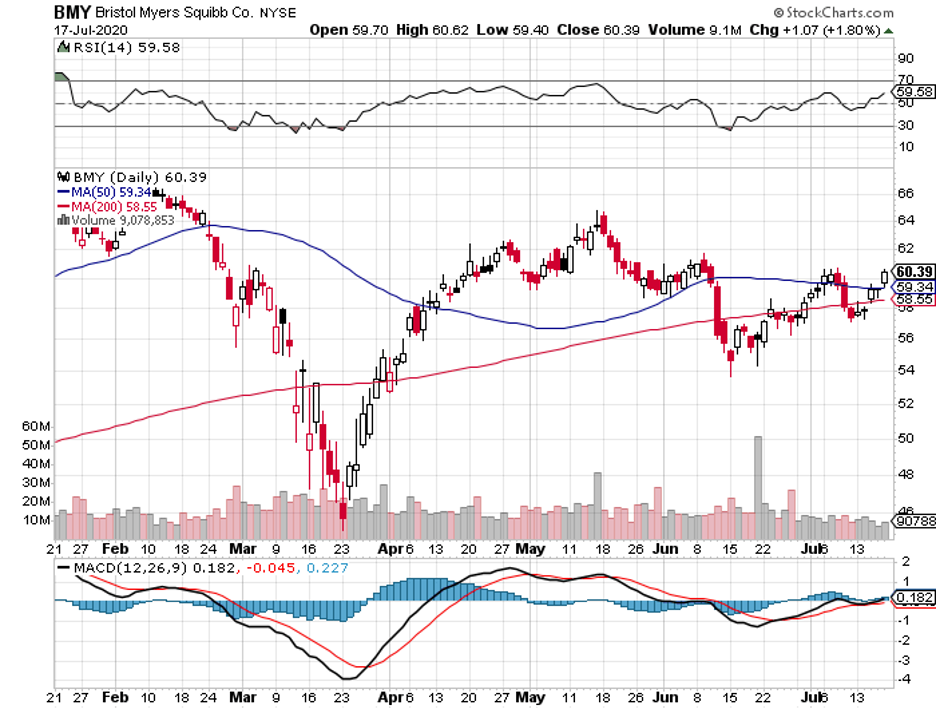
The fifth contender in the COVID-19 vaccine race is Merck (MRK).
While the rest of the developers are focusing on speed, Merck insists on taking its time before releasing their results. The company has made no announcement on its plans for human studies, with its leaders warning against accelerating the safety protocols.
At this point, there’s still no accurate way to determine the price of the COVID-19 vaccine. Nonetheless, investors are already smelling big money for this program.
A glaring example of how the COVID-19 crisis has pumped funds into biotechnology and healthcare companies is Moderna’s trajectory this year.
Prior to this pandemic, Moderna had a market capitalization of roughly $7 billion. Since February, though, this number has more than tripled its value at $23 billion.
While it is not the case for all the companies in the vaccine race, the excitement over it is understandable.
Let’s indulge in a bit of a back-of-the-envelope calculation.
If we base the estimates on the recent vaccines for diseases like meningitis B and the shingles, which cost somewhere between $300 and $400 for an entire course, then we can assume that the COVID-19 vaccine could fall near $500 per course.
That means vaccinating the entire population would rake in more than $150 billion to the company --- virtually all of its profit.
Inasmuch as that presents a lucrative opportunity, most of the leading companies already announced that they do not intend to make a profit off their COVID-19 programs.
These include Moderna, JNJ, and AstraZeneca. Meanwhile, Pfizer has yet to make that announcement.