Under normal circumstances, it would be unheard of for a biotechnology or pharmaceutical company to begin the construction of manufacturing facilities for any drug that has not gained approval from the US Food and Drug Administration (FDA).
However, the year 2020 has been anything but “normal.”
In fact, the US government has already released billions of dollars to companies working to create a COVID-19 vaccine well ahead of their candidates’ approvals by the FDA.
While we have yet to determine which vaccine candidates would work, the amount of money pouring into these programs give us a very real sense of the size of the vaccine market.
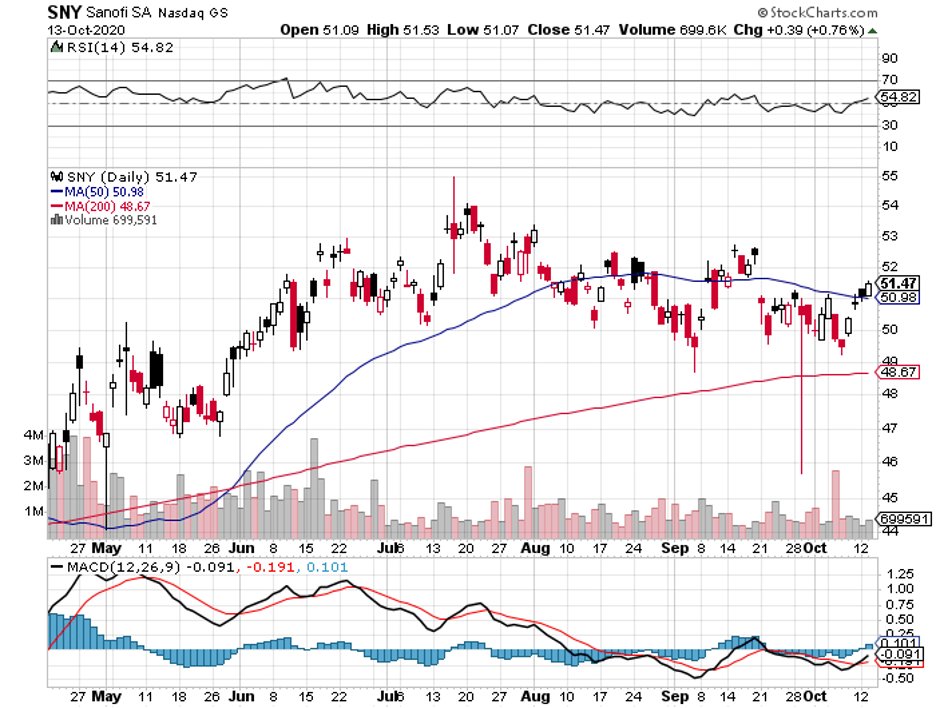
Among the companies working on a vaccine, Sanofi (SNY) and GlaxoSmithKline (GSK) emerged as early favorites.
Even without any candidate in late-stage trials, the two drug makers landed a $2.1 billion deal with the US government for their COVID-19 vaccine candidate in July.
This will cover 100 million doses initially, which would put the vaccine cost at $21 per dose.
If all goes well, the US government has the option to buy an additional 500 million doses of the Sanofi-GSK vaccine. The two companies are also negotiating terms with other countries particularly in Europe and Asia.
Sanofi is the lead partner in this program, with the company producing the COVID-19 vaccine itself. As for GSK, it will be adding an adjuvant which would boost the immune response.
Initial data from this study is expected to be released by December 2020, with the duo hoping to receive regulatory approval not later than June 2021.
The goal is to manufacture up to 1 billion doses annually from the time of its approval in 2021.
One of the reasons Sanofi and GSK candidate attracted attention despite the companies’ less aggressive timeline compared to competitors, like Moderna (MRNA), Pfizer (PFE), and AstraZeneca (AZN), is that it uses a protein-based technique already used in their flu vaccine called Flublok.
Using a tried and tested technology affords COVID-19 vaccine investors a safety net in case the newer and untested technologies of Moderna and Pfizer stumbles. For context, Flublok was approved by the FDA in 2013.
Aside from its COVID-19 vaccine program with GSK, Sanofi is working on a separate candidate with Massachusetts-based company Translate Bio.
This candidate, which uses mRNA technology, is expected to start human trials by November.
If all works out, Sanofi and Translate Bio estimate that they can produce 90 million to 360 million doses of this two-dose COVID-19 candidate in 2021.
Sanofi is no stranger to the vaccine market. In 2019, the company enjoyed a 4.8% year-over-year jump in its net sales and over 9% increase in the sales of its vaccines.
While Sanofi’s net sales slid by 4.9% in the first six months of 2020, the company still reported a healthy 9.2% growth in its earnings per share in the same period.
Thanks to its top-selling eczema drug Dupixent, the company’s specialty care segment rose by more than 17%.
In fact, the drug generated over $1 billion in sales in the first half of the year—a stunning 70% jump from its 2019 performance.
Riding this momentum, Sanofi has been aggressively adding new approvals for Dupixent and expanding its reach not only in the US but also in China.
Speaking of expansion, Sanofi recently completed a $3.7 billion acquisition of Principia Biopharma (PRNB) in August. This deal is a strong indicator that the company aims to focus more on its cancer and autoimmune sectors.
This also marks the second major acquisition of Sanofi in less than a year, with the company striking a $2.5 billion deal to acquire another cancer-focused biotechnology company Synthorx last December 2019.
Looking at the timeline of Sanofi compared to its competitor reminds me of the classic Aesop story, “The Hare and the Tortoise.”
However, the race for a COVID-19 vaccine is definitely not a winner-take-all scenario.
Sanofi and its partner GSK may look far behind the frontrunners, but these mega-companies have such extensive experience in developing and testing vaccines that they could easily close the gap in the next few months.
A successful COVID-19 vaccine would definitely be a gamechanger for Sanofi’s pipeline. The competition is stacked – several other resource-rich companies are also working on similar programs – and Sanofi’s candidates are nowhere near the finish line.
If Sanofi’s COVID-19 vaccine candidate is effective, however, there is really no good reason why it cannot snatch a piece of the pie.
Sanofi stock has not experienced any massive gains or losses since the pandemic started, and it probably will not make any investor get rich quick. But even without its COVID-19 vaccine candidate, this company is a tried-and-tested, reasonably priced value stock that any investor could simply buy and hold for decades.