Mad Hedge Biotech & Healthcare Letter
June 4, 2020
Fiat Lux
Featured Trade:
(MERCK’S BIG COVID-19 EXPANSION)
(MRK), (PFE), (GSK), (AZN), (MRNA)

Mad Hedge Biotech & Healthcare Letter
June 4, 2020
Fiat Lux
Featured Trade:
(MERCK’S BIG COVID-19 EXPANSION)
(MRK), (PFE), (GSK), (AZN), (MRNA)

Leading biotechnology and healthcare giant Merck (MRK), with a market capitalization of $203.75 billion, has been a low-key player during the pandemic.
Now, it finally reveals its grand plans via three major initiatives that aim to create a vaccine and design a novel antiviral against the coronavirus disease (COVID-19).
While Merck has been slow to take part in the COVID-19 war, it’s definitely making up for the lost time by announcing a promising acquisition and two collaborative projects in the works.
The first of the three deals is Merck’s acquisition of Vienna-based biotechnology company called Themis Bioscience. This small-cap biotech develops a range of vaccines and other therapies for infectious diseases.
One of Themis Bioscience’s pipeline candidates is a COVID-19 vaccine, which is a collaborative effort with the Institut Pasteur. Another promising candidate in Themis Bioscience’s pipeline is its late-stage work on a Chikungunya.
Through the acquisition, Merck will be able to access these works and hasten the development of the vaccine.
The second deal is Merck’s collaboration with a nonprofit scientific research group called International AIDS Vaccine Initiative (IAVI). This partnership aims to create a COVID-19 vaccine as well.
This will be a powerful collaboration since IAVI also received $38 million in funding from the US Health Department’s Biomedical Advanced Research and Development Authority (BARDA) to help them with their vaccine development initiatives.
Apart from IAVI, BARDA also awarded over $2 billion in funding to other vaccine developers like AstraZeneca (AZN), Phlow, and Moderna (MRNA).
Together, IAVI and Merck aim to optimize the latter’s recombinant vesicular stomatitis virus (rVSV) technology which has been used for Merck’s Ebola vaccine called Ervebo.
The third deal is Merck’s partnership with privately held Ridgeback Biotherapeutics, a Miami-based biotechnology company founded in 2018.
The collaboration intends to develop an oral antiviral treatment, dubbed as EIDD-2801, which can be used for COVID-19.
So far, this developmental drug had been proven safe in a trial with healthy volunteers. Clinical testing for COVID-19 patients has already commenced.
Under the terms of the deal, Merck will own exclusive global rights to EIDD-2801.
The giant biopharma will take charge of the clinical development, manufacturing, and regulatory procedures. In exchange, Ridgeback received an undisclosed amount in upfront payment and milestones. The smaller biotechnology company will also be entitled to a share of net proceeds from the COVID-19 treatment.
Prior to these deals, investors have been curious as to why Merck was missing in action amid the government’s “Operation Warp Speed” for COVID-19 treatments and vaccines.
With this triple play, Merck has signaled that it’s also going all-in on this pandemic and will pull out all the stops to be on the same level as the efforts from other major biotechnology and healthcare players like GlaxoSmithKline (GSK), Pfizer (PFE), and AstraZeneca (AZN).
The strategic moves from this healthcare giant clearly underscore the incredible demand for any vaccine that actually survives the R&D gamut, as every nation across the globe frantically looks for a vaccine to boost their people’s immunity.
While companies such as CanSino (HKG:6185) and Moderna go neck to neck to take the lead in the clinical race, we all know that two companies cannot handle the production of a vaccine for the entire world -- offering Merck a slot at a place in these chosen group of companies.
Outside its COVID-19 efforts, Merck recently chalked up another win for its blockbuster melanoma drug Keytruda. This time, the top-selling medication expanded its use to advanced colon cancers.
Based on key findings, Keytruda lowered the risk of the disease’s progression or even death by 40% compared to chemotherapy.
Results show that the tumors of patients given Keytruda did not grow for 16.5 months. In comparison, chemotherapy patients experienced tumor growth in 8.2 months.
Even prior to the expansion of its indications, Keytruda sales have continued to make headway.
In the first quarter of 2020, sales of this cancer drug reached $3.3 billion, showing off a whopping 45% year-over-year jump.
With this new addition to Keytruda’s indications, sales of this drug is expected to climb even higher this year.
Apart from that, Merck’s HPV vaccines, called Gardasil and Gardasil 9, performed well in the first quarter as well as sales of both HPV vaccines increased by 31% to reach $1.1 billion.
Another strategic effort for Merck is delving into drug development focused on neurodegenerative diseases like Alzheimer’s, Parkinson’s, and Huntington’s disease.
This initiative was kicked off by Merck’s move to buy a GSK startup, called Calporta Therapeutics, for $576 million in 2019.
It was followed by forging a two-year partnership with Almac Discovery this year. Apart from neuro-related diseases, the two companies are looking into developing therapies for cancer and viral diseases as well.
To further boost its pipeline, Merck completed a deal with Taiho Pharmaceuticals and Astex Pharmaceuticals worth $50 million in upfront payment plus incentive payments of up to $2.5 billion.
The company is also working on spinning off its "Women's Health, Trusted Legacy Brands, and Biosimilars” products into a brand new company with a focus on oncology and vaccines as well as animal health. If things go smoothly, the spinoff should be done by the first half of 2021.
Overall, Merck has proven itself as a stable dividend stock that investors can simply buy and hold for years.
The biotechnology company has managed to post a profit every year for the past decade, actually hitting double-digits most of the time within that period.
This is a trend observed in Merck’s first quarter report as well. The company posted $3.2 billion in profit on sales worth $12.1 billion. This represents a respectable 27% profit margin.
Mad Hedge Biotech & Healthcare Letter
May 28, 2020
Fiat Lux
Featured Trade:
(ASTRAZENECA’S WASHINGTON FREEBIE)
(AZN), (MRK), (PFE), (JNJ)
The coronavirus disease (COVID-19) has pushed people to gamble on unproven and untested products for as long as these can offer even just a shred of hope.
Call it the grasping for straws strategy.
The latest biotechnology company granted this kind of confidence is AstraZeneca (AZN).
The U.S. Department of Health and Human Services (HHS) recently announced its decision to provide the cardiovascular heavyweight up to $1.2 billion in funding to speed up the company’s progress on AZD1222. The problem is that the vaccine has not been proven to work.
You would think that if the US government was going to blow $1.2 billion on an unproven vaccine at least it would be on an American company. That is not the case. Pre pandemic, a drug at this stage of development would not have earned the time of day.
The experimental vaccine is a gene therapy that AstraZeneca has been working on alongside Oxford University. Aside from the funding, HHS also committed to supporting the clinical trial for this vaccine which would involve 30,000 participants.
In return, the biopharmaceutical company has agreed to send up to 300 million doses of the vaccine to the US, with the first shipment expected to be sent before 2020 ends. AstraZeneca will also provide 100 million doses to Great Britain.
Before getting too excited on this news though, it’s critical to note that AZD1222 is not yet proven effective against COVID-19.
This proposed vaccine from Oxford is created from a weakened version of a common cold virus. It was then genetically altered to disable it from growing in humans. So far, over 1,000 individuals already received this vaccine during an early stage test that started in April.
The plan is for the two companies to use the same technique that the Oxford University researchers applied to fight the Middle East Respiratory Syndrome (MERS).
Based on the results of a small clinical trial, this approach managed to prevent the transmission of MER-CoV among the patients. The goal is to replicate this success and halt the spread of COVID-19.
With AstraZeneca securing a manufacturing capacity of 1 billion doses, the first deliveries of the vaccine are estimated to start by September.
Apart from the US and the UK, AstraZeneca is also tapping the Serum Institute of India along with other potential collaborators to boost the production of the COVID-19 vaccine.
While all these updates sound promising, it’s undeniable that choosing which stock to invest in this chaotic market is not for the faint of heart.
The pandemic has clouded the earnings landscape of practically all companies across the board for at least the rest of 2020, with the uncertainty possibly spilling into 2021.
So far, several vital members of various industries have already sought unprecedented levels of aid from the state. A glaring example of this is the ailing airline industry, which has been receiving the most help from governments across the globe.
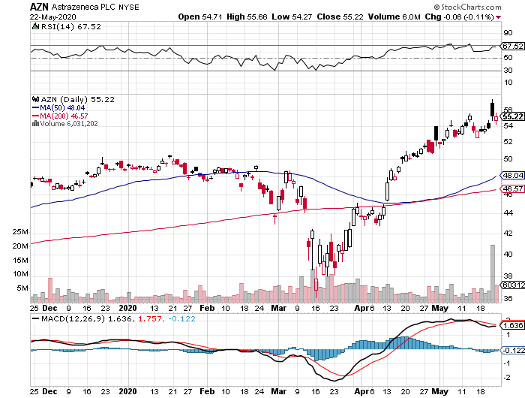
Meanwhile, AstraZeneca’s shares are up nearly double from the March bottom.
Looking at its financial history, it can be said that AstraZeneca’s ability to swim against the current is due in large part to its top-quality clinical pipeline.
In 2018 alone, the company received a whopping 23 regulatory approvals in various fields including oncology, cardiovascular, and kidney disorders.
The latest product to boost AstraZeneca’s already potent pipeline is its collaboration drug with Merck (MRK) called Lynparza.
Previously approved as a treatment for ovarian and breast cancer, Lynparza is now approved as medication for prostate cancer as well.
This label expansion allows AstraZeneca and Merck to go head to head against Johnson & Johnson’s (JNJ) Zytiga and even Pfizer (PFE) and Astellas Pharma’s Xtandi.
In 2019, Lynparza’s annual sales increased by 85% primarily because of label expansions. The drug is anticipated to show a surge in its 2020 sales as well thanks to this recent approval.
However, the best is yet to come for AstraZeneca.
While the company has put on hold a couple of its clinical trials, reports show that it has many late-stage data readouts up its sleeve. Hence, investors should be on the lookout for these announcements in the next 24 months.
Due to all the new growth products and label expansions, AstraZeneca’s top line is projected to jump by 13.7% in 2021, making it one of the fastest forecasted growth rates in the industry at this point.
For even more good news, this biopharmaceutical powerhouse disclosed that it has no intention of revising its annual guidance despite the pandemic. This announcement is tangible proof of the economic staying power of AstraZeneca’s portfolio.
AstraZeneca stock is not exactly cheap though. With its recent developments and track record, the company has definitely gained a following in the biotechnology and healthcare sector. Nonetheless, AstraZeneca has proven that it deserves its top-shelf valuation.
Mad Hedge Biotech & Healthcare Letter
May 19, 2020
Fiat Lux
Featured Trade:
(PFIZER’S LATEST COVID-19 VACCINE ENTRY)
(PFE), (BNTX), (MRNA), (INO), (CTLT), (SVA), (EBS), (MYL)
Clearly, the long-term solution to this health crisis, and possibly the only hope we have to returning to “normal,” is a safe and effective vaccine.
Companies and health experts around the world have stepped up to that challenge, with investors eagerly anticipating the stocks of the businesses to successfully deliver a vaccine to catapult in value overnight.
This is one of the driving forces behind Pfizer’s (PFE) relentless pursuit of a coronavirus vaccine.
Here’s a quick recap of where Pfizer was before this major announcement.
Pfizer was first recognized as an aggressive player in the vaccine race when the healthcare giant partnered with German biotechnology company BioNtech (BNTX).
After months of working together, Pfizer announced that it aims to produce 10 million to 20 million doses of COVID-19 vaccine by the end of 2020.
So far, Pfizer is testing at least four distinct variations of its vaccine called BNT162. The trials will test roughly 360 individuals, with the study expanding to involve thousands of volunteers if one or two variations of the vaccine indicate progress.
Conclusive data will be available in June or July this year. Meanwhile, Pfizer’s coronavirus vaccine candidate, co-created with BioNtech, is projected to be ready for launch by October.
In an effort to make room for the production of BNT162, Pfizer decided to outsource the production of some of its own branded products to various manufacturers such as Catalent (CTLT).
This move means that instead of paying contract manufacturers to produce millions of doses of a vaccine that might fail to even leave the warehouse, Pfizer has taken it upon itself to produce BNT162 in its own facilities.
According to the company’s estimates, it will cost approximately $150 million to produce BNT162. Since Pfizer is using its own facilities, it could jumpstart the distribution of up to 20 million doses of COVID-19 vaccine even before 2020 ends.
This move to ramp up the manufacture of an experimental drug candidate is a surprising gamble for Pfizer. However, the possibility of having millions of doses of this potential vaccine ready to ship at a moment’s notice could make it a worthwhile risk.
In terms of competition, Pfizer is racing against several biotechnology companies searching for a COVID-19 vaccine in the US and abroad.
One of them is Moderna (MRNA), which has a $19 billion market cap and funding access worth $2.4 billion including government endowment.
Moderna collaborated with Lona (OTC: LZAGY), which is an international chemical manufacturer, to scale up its production power.
Apart from this, smaller biotechnology companies like Inovio Pharmaceuticals (INO) and Novavax (NVAX) are involved in the COVID-19 vaccine race as well.
Inovio is backed by its history of vaccine research on the swine flu outbreak in 2009 and the 2013 avian flu.
Novavax, which has a modest market cap of $82.2 million, received government funding worth $4 million to help the company move forward with clinical trials.
Additional financial support was also sent by the Coalition for Epidemic Preparedness Innovations. In terms of manufacturing, Novavax has been working with Emergent BioSolutions (EBS) to meet production demands.
Outside the US, two of the frontrunners are Chinese companies CanSino Biologics and Sinovac Biotech (SVA).
The stocks of various micro-cap companies have been on the news since the COVID-19 vaccine race started. Several of these smaller firms used their newfound popularity to boost their stock price and generate additional capital to fund their operations.
I think there are several biotechnology and healthcare companies that warrant following. However, there remains a dearth of data on these companies working on the COVID-19 vaccine. Choosing the best stock from these names at this point demands too much guesswork, an investment strategy I have never endorsed.
The harsh reality is that most of these smaller companies will most probably never manage to get a program off the ground and into a conclusive efficacy trial. The main reasons are limited capital, restricted bandwidth, and lack of will to move forward.
Small companies, particularly in the biotechnology and healthcare sectors, typically lack the money and manpower to efficiently run a program without sacrificing the rest of their R&D efforts. For those companies that manage though, the pace will likely be too slow to actually merit a meaningful place in the market.
Investors looking to invest in the surging COVID-19 vaccine space should turn to companies that hold the greatest odds of success. That means larger and more established companies with global testing, regulatory, and manufacturing capacities.
This is not to dissuade anyone from taking a dip into the small-cap companies pool though.
Rather, I would recommend to simply keep these biotechnology companies on your watch list and see how the situation develops. After all, these are decent stocks on their own right.
Nonetheless, it’s still too early to tell how their long-term business models look like outside the search for a coronavirus vaccine.
In comparison, Pfizer has a proven track record of being a great investment. The company has been showing off a decent dividend growth for 10 consecutive years, reporting an annualized dividend worth $1.52 per share.
More importantly, this biotechnology and healthcare company is showing no signs of slowing down anytime soon. In 2019 alone, Pfizer introduced six new drugs on the market and shared that it has 95 more in its pipeline.
Keep in mind as well that Pfizer’s current price of roughly $37 per share -- a far cry from its 52-week high that reached $44.56 -- is significantly lower than the industry average at the moment. For a stock that presents such a wealth of opportunities, Pfizer offers significant value to its investors.
Mad Hedge Biotech & Healthcare Letter
April 30, 2020
Fiat Lux
Featured Trade:
(GILEAD SCIENCES GOES BALLISTIC ON REMDESIVIR TRIAL)
(GILD), (PFE), (JNJ)
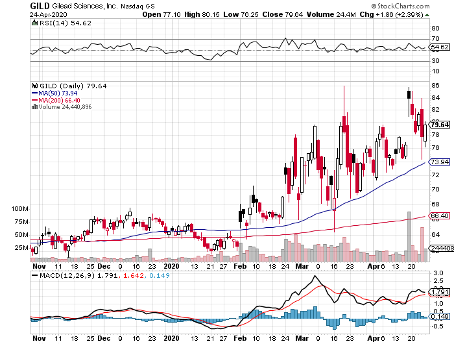
Gilead Sciences (GILD) is aggressively pushing to bring the coronavirus disease (COVID-19) to its proverbial knees before this year ends.
The ongoing coronavirus disease (COVID-19) pandemic has brought about substantial disruptions to the world economy, not to mention the devastating losses it caused families watching their loved ones succumb to this deadly virus.
Apart from Gilead, other biotech giants like Pfizer (PFE) and Johnson & Johnson (JNJ) are also hard at work looking for a COVID-19 cure.
Luckily, reports indicate that they may finally see a light at the end of the tunnel as one experimental treatment showed promising efficacy for fighting the health crisis.
Based on the contextual analysis of the leaked information on the clinical trials conducted by Gilead, the biotech company’s decision to bet on Remdesivir as a probable COVID-19 treatment could pay off soon.
At this point, Remdesivir is still under investigation in several Phase 3 clinical trials. These involve more than 2,400 participants scattered in 152 clinical sites.
One of these locations is the University of Chicago Medical Center, where 125 patients who tested positive for COVID-19 are treated every day with infusions of Remdesivir. Out of these individuals, only two deaths were reported with the majority already discharged.
Based on this subgroup alone, the fatality rate among the tested subjects is 1.6%.
Although Remesivir’s results still need further validation particularly in terms of adding a placebo arm in the clinical tests, the initial findings are already quite impressive. For context, data from John Hopkins University revealed that the fatality rate in the entire United States is roughly 4.69%.
Apart from that, another key detail points to the high probability of Remdesivir’s efficacy against COVID-19.
Among the 125 patients who underwent the treatment, 113 of them experienced severe symptoms.
As explained by the World Health Organization, the vast majority of those classified as severe cases involve the elderly and the immunocompromised. In one study, the infection death rate of individuals in this category fall somewhere between 1.93% up to 7.8%
Reassessing Remdesivir’s results from this perspective, we finally understand the excitement surrounding the drug’s efficacy despite the lack of a placebo trial.
In terms of questions on Remdesivir’s economic potential, we can take a look at past respiratory outbreaks like the H1N1 in 2009 and the 1918 Spanish flu for guidance.
Despite “flattening the curve,” at the time, both diseases had resurgences that reached second and third waves after the initial outbreaks were contained.
Combined, the H1N1 and the Spanish flu infected roughly 24% to 33% of the entire global population prior to subsiding for good.
Hence, high demand for Remdesivir will be expected even after the world manages to contain the first COVID-19 outbreak.
What does this mean for Gilead investors?
Remdesivir results are expected to come back positive by the end of April. With the FDA’s Coronavirus Treatment Acceleration Program, the drug is estimated to gain approval in a few months' time.
If successful, Remdesivir is projected to rake in more than $1 billion in sales throughout the coronavirus outbreak period. This estimate is based on the sheer number of people infected and are potentially at risk.
The estimated sales figure is also based on the assumption that Gilead can produce enough Remdesivir supply to treat up to 500,000 patients and that the drug will cost roughly $2,000 for a single-course treatment.
Adding Remdesivir in its lineup, Gilead has adjusted its 2020 revenue guidance to surpass $22 billion with sales growing by more than 4%, thanks to this potential COVID-19 drug alone.
However, Gilead offers more than a promising COVID-19 treatment.
The biotech giant prides itself of a strong lineup, showing off a particular dominance as the market leader for HIV treatments.
Its top HIV drug Biktarvy saw a whopping 300% increase in its sales last year, reaching $4.7 billion in 2019 alone -- and it still hasn’t reached its peak.
Analysts noted that Biktarvy has more room to grow in the next years, with the HIV drug anticipated to continue serving as Gilead’s significant growth driver until 2033.
Another HIV market leader is Descovy, which is set to be the preferred choice among 40% to 45% of patients by the end of 2020.
Despite these promising developments, Gilead stock is still pretty cheap.
To date, this biotechnology company is trading at 13.2 times forward earnings with a measly PEG ratio of 0.3.
At this price -- and considering the company’s strong portfolio and pipeline candidates -- investors on the lookout for biotech exposure but are worried about the consequences of the COVID-19 pandemic should definitely add Gilead into their core holdings.
Mad Hedge Biotech & Healthcare Letter
April 9, 2020
Fiat Lux
Featured Trade:
(A SLIVER OF HOPE FOR CORONAVIRUS)
(MYL), (NVS), (BAYRY), (PFE)
To this day, there’s still no solid proof that any drug can treat or prevent infection with the deadly coronavirus. Faced with an exploding pandemic that brings an alarming death toll, the public is eager -- desperate -- for a sliver of hope and some news regarding discoveries of COVID-19 treatment.
Lately, the drug that has been gaining so much attention is hydroxychloroquine. This is primarily thanks to Trump’s endorsement, with the president going as far as labeling it a “miracle” drug.
By now, we’ve become all too familiar with the story behind this “miracle” drug.
Trump was watching TV the night before and saw a feature about a Michigan woman who was suffering from COVID-19 for 12 to 14 days. Her suffering was so intense that she felt she would die anytime soon. One night, she asked her husband to find hydroxychloroquine.
Four hours after taking it, she felt better and eventually recovered.
While health experts are still waiting for conclusive evidence on the drug’s efficacy, this story inspired Trump to urge the public to try it as well.
Aside from describing it as a potential cure, Trump is also recommending hydroxychloroquine as a preventive measure for health workers. His point is that there’s really nothing to lose here. After all, the drug has been used for decades so “it’s not going to kill anybody” compared to completely novel treatments.
In fact, he has been so intent in using hydroxychloroquine to cure COVID-19 patients that he ordered 29 million doses added to the government’s cache of medical supplies.
Just what is hydroxychloroquine?
This is a prescription drug approved to treat malaria decades ago. It can also be prescribed to treat autoimmune diseases such as lupus and rheumatoid arthritis. It’s called by its brand name Plaquenil as well.
Is it really effective to treat COVID-19?
The answer remains unclear. However, there are a couple of studies that point to promising results.
One is a laboratory study using cultured cells. In this research, it was found that chloroquine has the ability to prevent the coronavirus from invading the cells. Obviously, blocking the virus means protecting the body from the illness.
However, scientists issued a word of caution about this.
They reminded us that the drugs that work well in killing off viruses in petri dishes or test tubes do not necessarily translate to the same results in the human body.
As for hydroxychloroquine, studies showed that it can’t prevent or cure influenza and other viral diseases.
This doesn’t mean that hydroxychloroquine is useless as a COVID-19 treatment.
It just shows that more trials are needed to determine its actual effect. Several studies have been launched to figure out the answer to this.
In Detroit alone, there will be 3,000 patients set to participate in the trial to come up with a formal and conclusive study on hydroxychloroquine.
In a nutshell, what the health experts are saying is that the celebration might be a tad premature.
So this leads to a lot of investors to wonder which companies stand to benefit if hydroxychloroquine gets approved as a COVID-19 treatment.
Probably no one.
Keep in mind that this is an old drug, which came to the market sometime in the 1940s. Hence, it’s highly unlikely for it to become a blockbuster drug for any company.
Right now, several companies are already making it, including Novartis (NVS) and Bayer (BAYRY).
However, investors interested in buying cheap biotech stocks might be interested in generic drug maker and Mylan (MYL) are also in the running.
When Trump started touting the effects of hydroxychloroquine on COVID-19 patients, Mylan immediately restarted its production of the tablets.
The company aims to have the drug available in the market by mid-April, targeting up to 50 million tablets for over 1.5 million people.
Like I said, hydroxychloroquine isn’t going to be a high-selling drug for any company.
Nonetheless, this could provide the much-needed momentum for Mylan as its investors start to lose confidence in the company.
With the company back in the spotlight, it can easily redirect everyone’s attention to its upcoming merger with Pfizer’s (PFE) Upjohn unit to form a new company called Viatris.
This combined company will hit the ground running as it buys two assets from Pfizer.
One will be Meridian, which is the maker of EpiPen along with other auto-injectible treatments. The second is Mylan-Japan, which has been the generics collaboration unit of Pfizer and Mylan since 2012.
Both units recorded $598 million in annual revenue in 2019.
Viatris’ portfolio will also include a number of top-selling products like erectile dysfunction and pulmonary arterial hypertension Viagra and arthritis Celebrex.
The lineup will even feature the blockbuster cholesterol drug Lipitor, which generated more than 2 billion in sales last year alone.
According to the terms of the deal, Pfizer shareholders will own 57% of Viatris while Mylan shareholders get 43%.
Due to the upcoming merger, Pfizer went ahead and upped the 2020 guidance for Upjohn’s revenue from the $7.5 billion and $8 billion range to $8 billion and $8.5 billion.
The Viatris spin-off is expected to be completed by mid-2020.
For years, Mylan has been plagued with numerous issues like pricing concerns and even lawsuits.
Hence, this merger with Upjohn is considered a crucial turning point for Mylan. It represents a fresh beginning from this previously embattled stock.
Legal Disclaimer
There is a very high degree of risk involved in trading. Past results are not indicative of future returns. MadHedgeFundTrader.com and all individuals affiliated with this site assume no responsibilities for your trading and investment results. The indicators, strategies, columns, articles and all other features are for educational purposes only and should not be construed as investment advice. Information for futures trading observations are obtained from sources believed to be reliable, but we do not warrant its completeness or accuracy, or warrant any results from the use of the information. Your use of the trading observations is entirely at your own risk and it is your sole responsibility to evaluate the accuracy, completeness and usefulness of the information. You must assess the risk of any trade with your broker and make your own independent decisions regarding any securities mentioned herein. Affiliates of MadHedgeFundTrader.com may have a position or effect transactions in the securities described herein (or options thereon) and/or otherwise employ trading strategies that may be consistent or inconsistent with the provided strategies.
