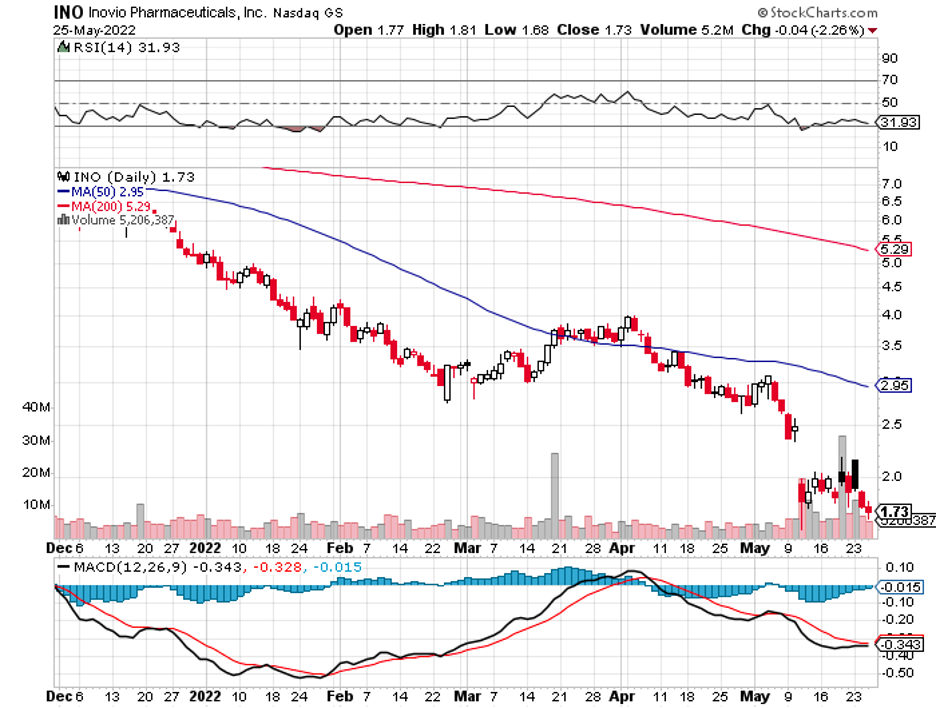
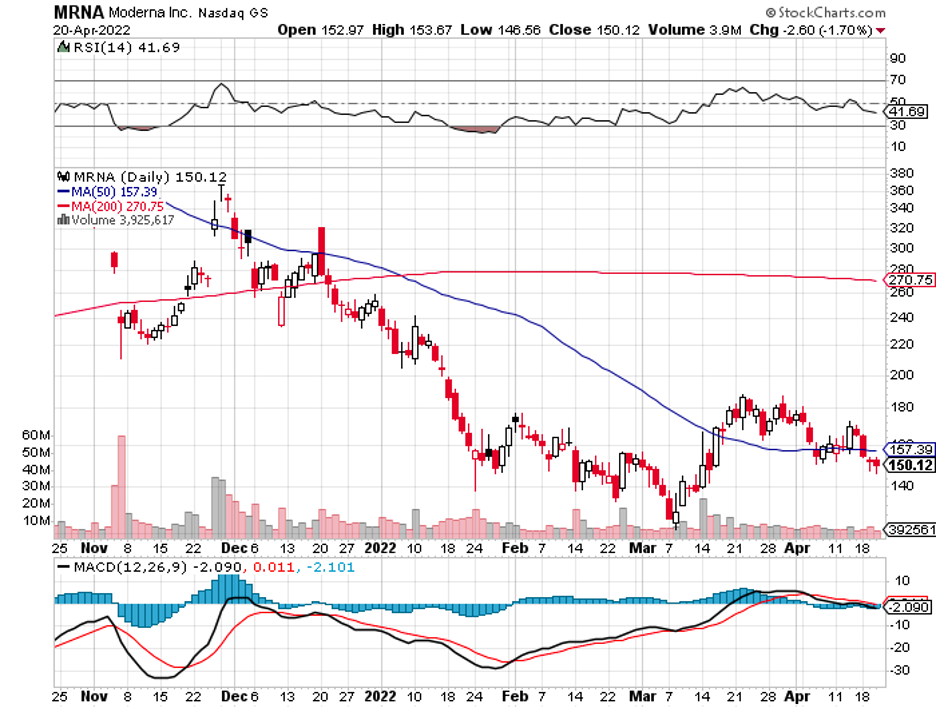
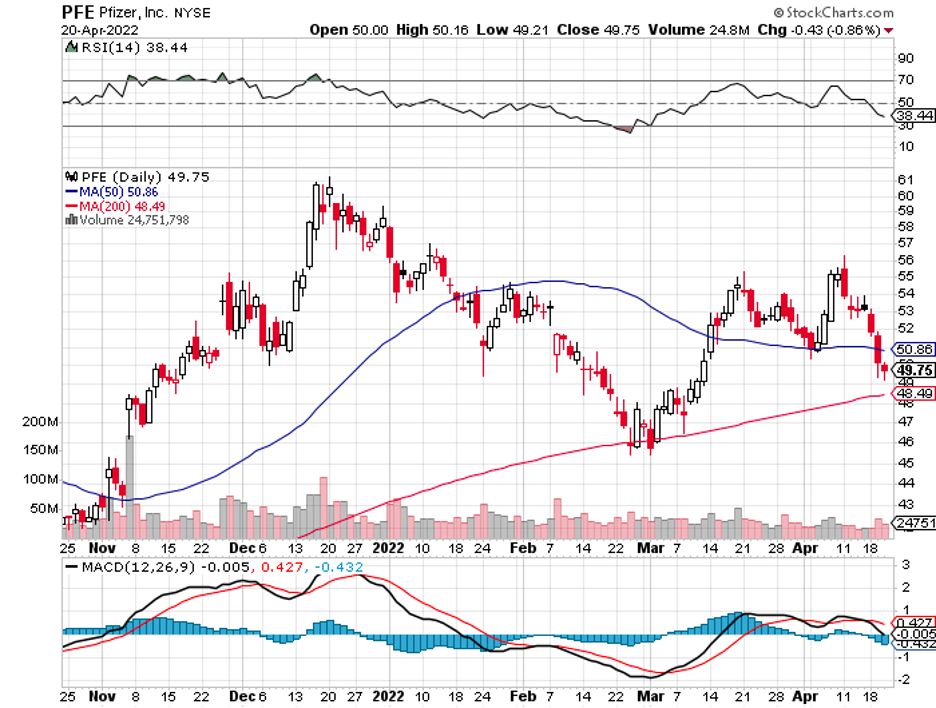
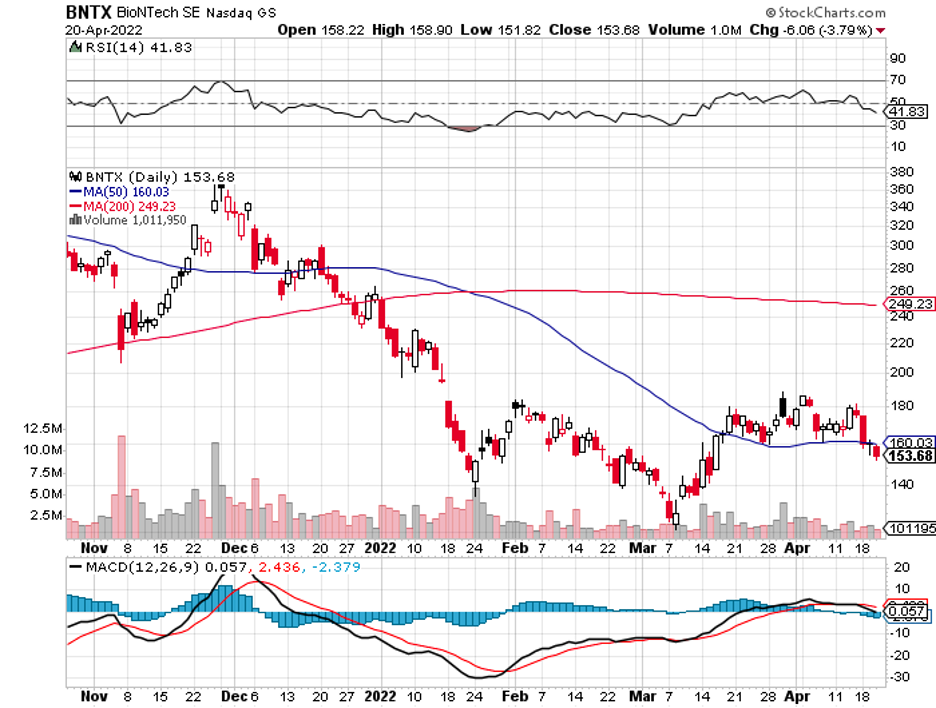
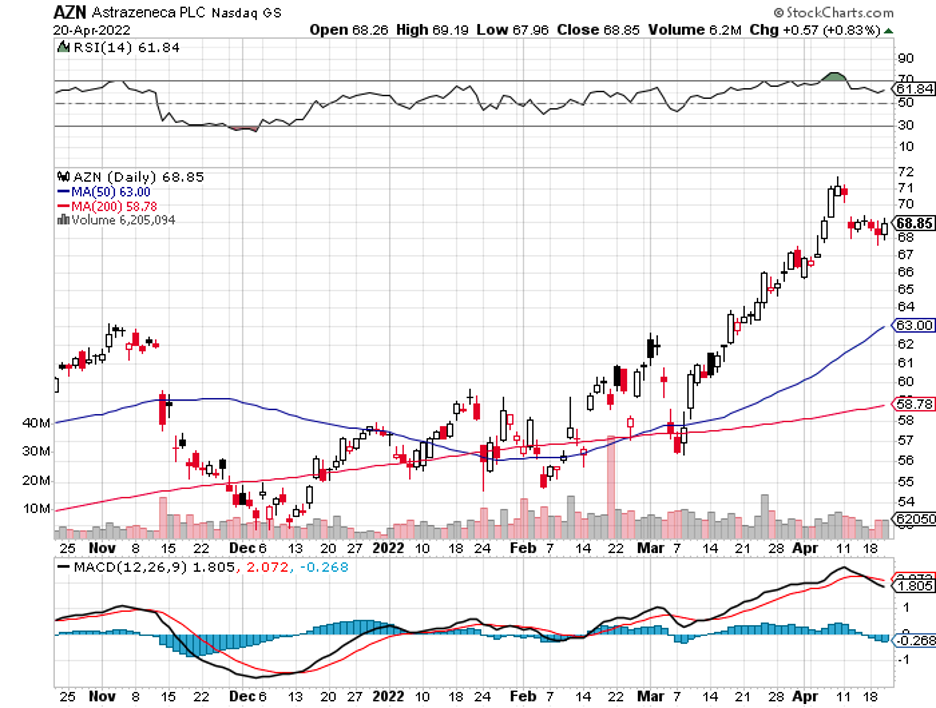
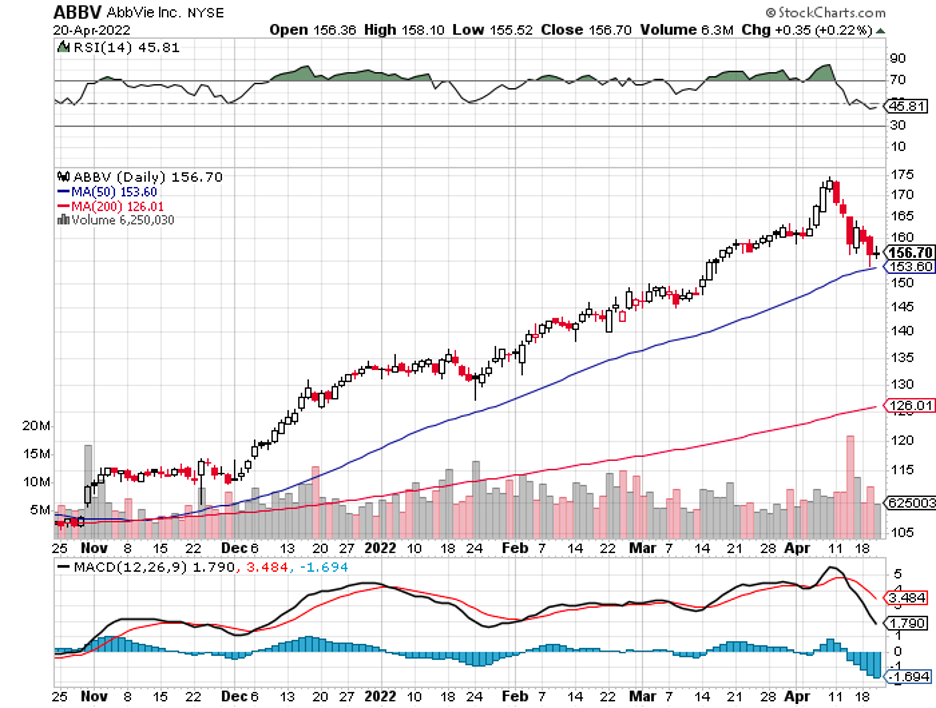
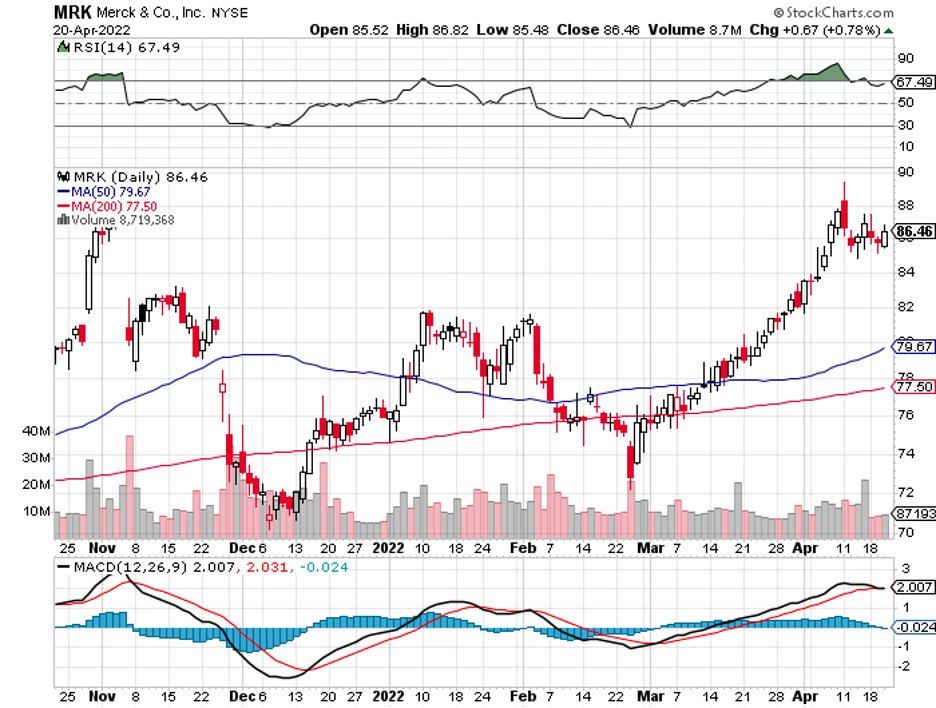
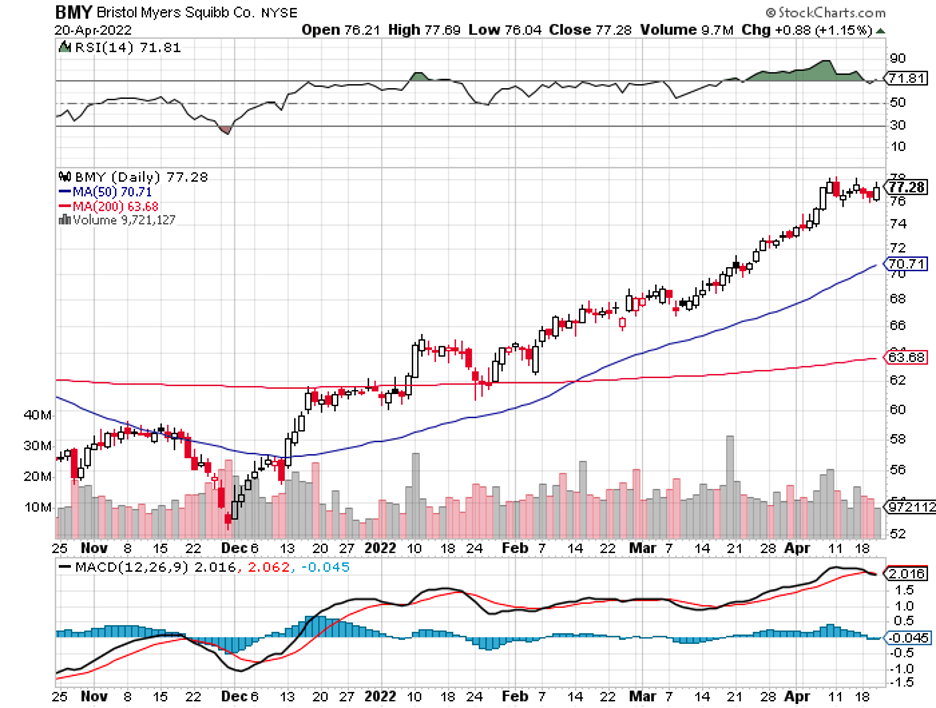
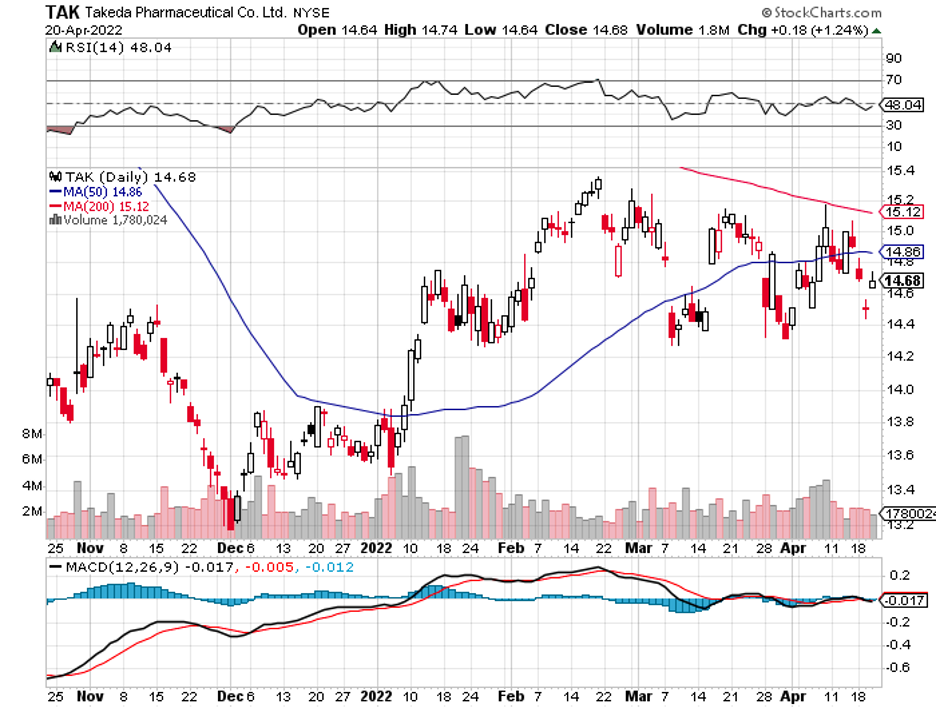
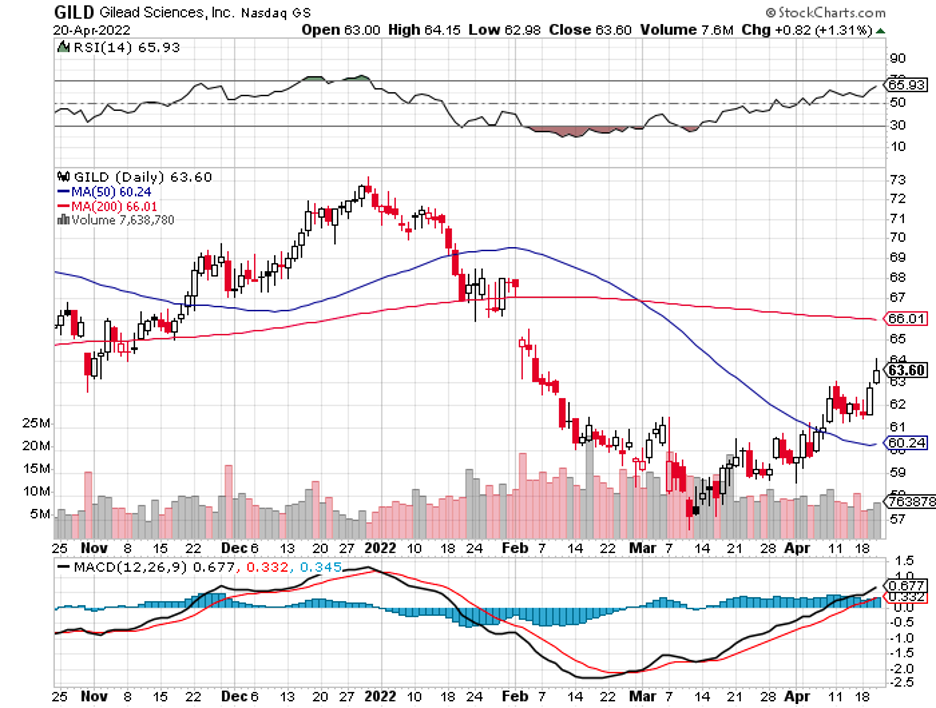
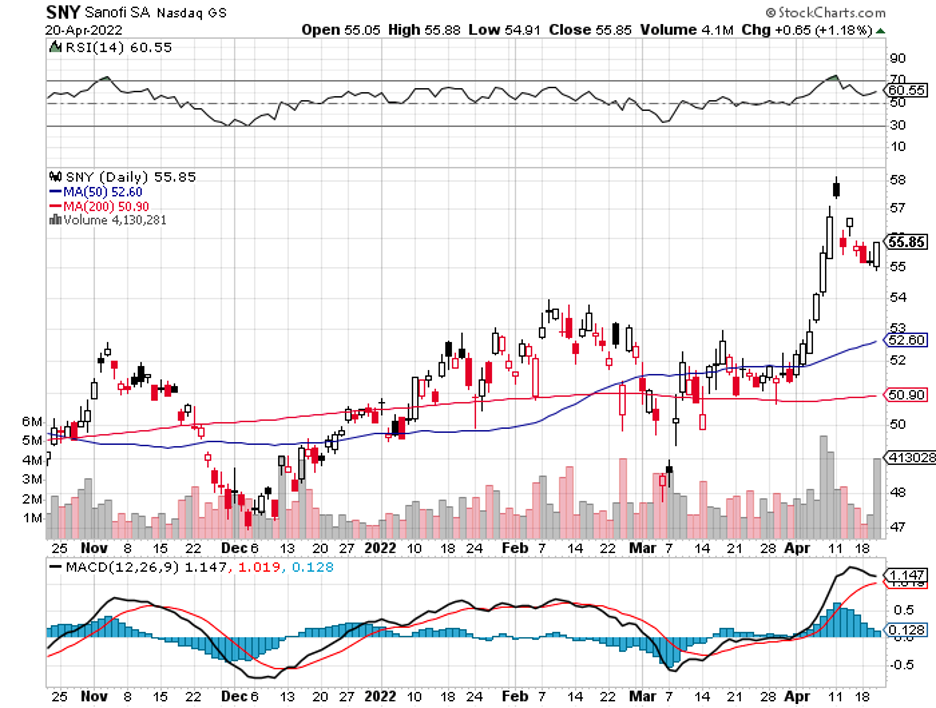
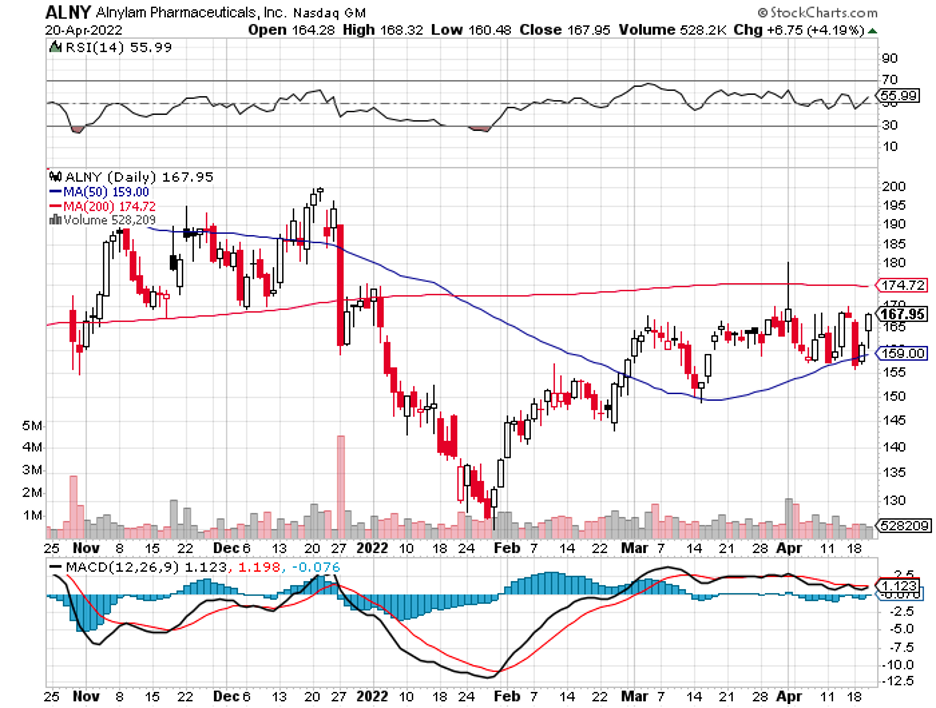
Biotechnology stocks have been rallying since mid-June, and it looks like the sector doesn’t have plans of stopping anytime soon.
The SPDR S&P Biotech ETF (XBI), which keeps track of the segment, has been up by 32.5% since the second half of 2022—a period that saw the S&P 500 rise by only 11.4%.
Nonetheless, doubts still linger in terms of how long this sector’s bull run will last. There are also questions on whether the recent shift in market sentiment indicates a substantive change or merely a momentary blip.
News about the biotech industry has been leaning towards the positive in the past months, and hopes for its recovery were bolstered by the much-discussed potential acquisition of Seagen (SEGN) by Merck (MRK).
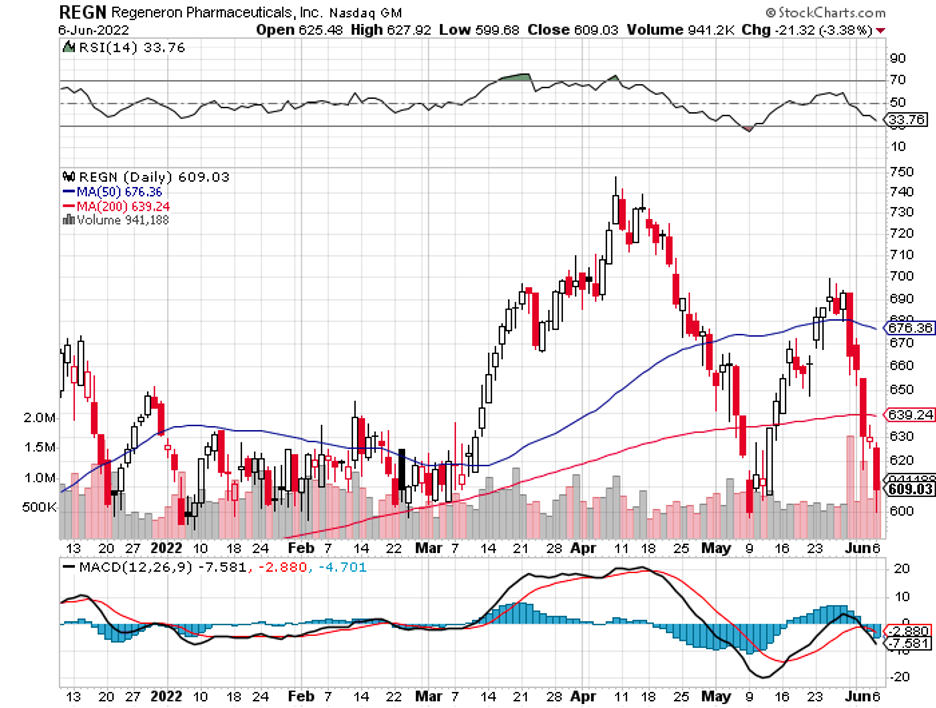
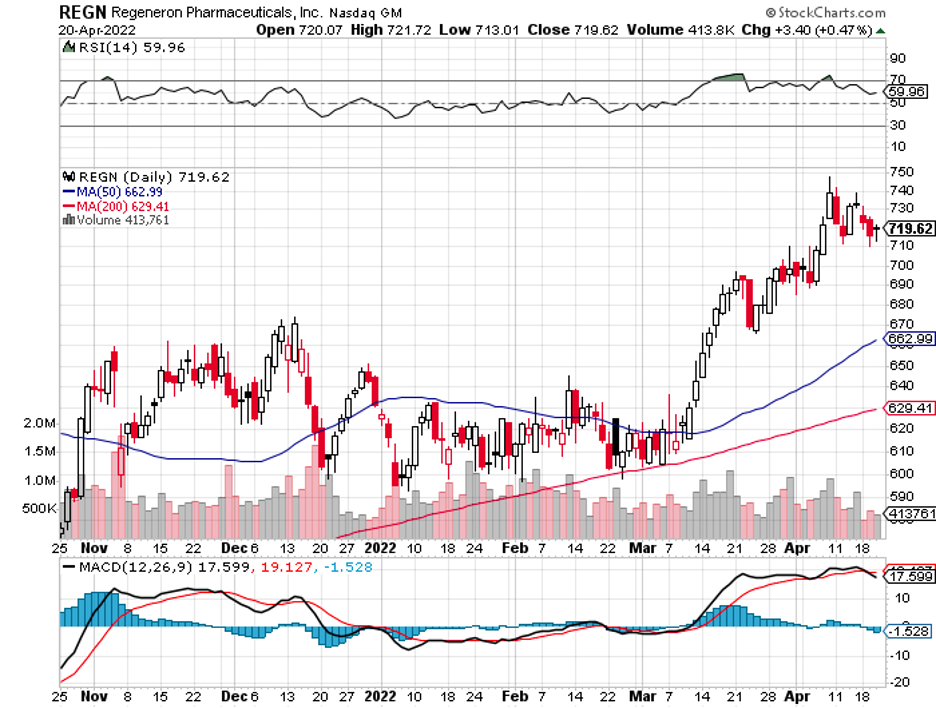
The strong earnings reports of Regeneron (REGN) and Gilead Sciences (GILD) also added to the overall positivity of the sector.
Meanwhile, another big mover in the biotechnology world appears to be gearing up for a major move soon.
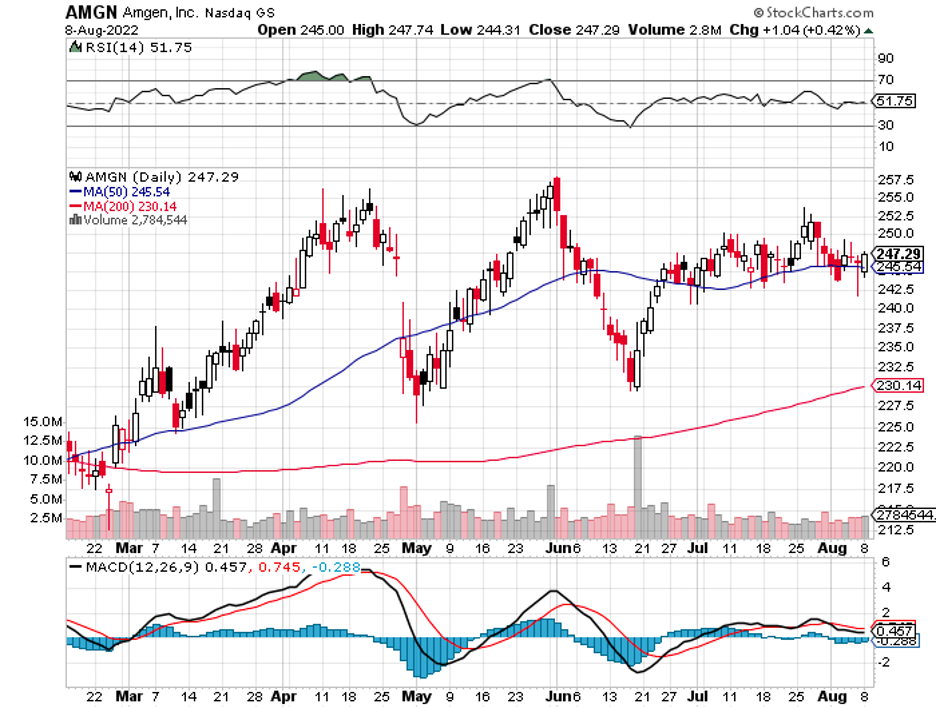
Amgen (AMGN) recently announced its plans to acquire ChemoCentryx (CCXI) for $3.7 billion.
This all-cash acquisition works out to roughly $52 per share and a whopping 115% premium to ChemoCentryx’s price.
ChemoCentryx is mostly known for its autoimmune disorder pipeline. In 2021, the company received FDA approval for Tavneos, which targets a relatively rare autoimmune condition called ANCA-associated vasculitis.
In the first quarter of 2022, Tavneos delivered $5.4 million in sales.
The announcement boosted ChemoCentryx’s shares to skyrocket by 108.4% while Amgen shares remained flat. However, this jump isn’t all too surprising.
The company getting acquired records a jump in stock price after the announcement because the acquirer typically pays a premium for the deal. It’s a strategic move since the higher the premium, the better the chances that the shareholders will approve the acquisition.
If all goes well, this acquisition is expected to be completed by the fourth quarter of 2022.
This move is a good indicator of Amgen’s response to its problem of stagnation. Over the years, this biotech giant has been seemingly left behind in churning out innovative treatments.
Pursuing a promising company like ChemoCentryx is an excellent way to diversify its pipeline and reignite growth.
The deal is especially promising in light of the company’s major setback in 2020 when the Phase 3 clinical trial for its heart failure drug fell short of delivering the promised results.
While issues with new products aren’t exactly new, particularly in the biotechnology sector, Amgen’s failure made investors skittish and led to selloffs.
However, Amgen was not deterred. After all, the setback came following decade-long progress leading up to 2020 when the company’s revenues steadily rose from $15 billion to $23 billion.
In the end, Amgen was still able to surpass its projected revenue to hit the $25 billion mark in 2020 thanks to its strategic move to acquire Otezla from Bristol Myers Squibb (BMY).
By 2021, Amgen shared that its 2020 results were up 9%. Last year, the company ignited some momentum and managed to raise its earnings from the year before to $26.2 billion.
Despite these efforts, the company still struggled with organic growth. This is perhaps why it has been aggressive in pursuing multiple revenue streams via M&A to find more ways for multiple expansions.
Part of this plan is the 2021 acquisition of Five Prime Therapeutics for $1.9 billion and Teneobio for $900 million.
Given the deals last year, investors didn’t truly expect Amgen to deliver more growth in 2022. This is possibly why the company’s shareholders were a bit surprised by the new acquisition.
However, this deal with ChemoCentryx will grant Amgen access to a slew of orally administered treatments not only for autoimmune diseases and inflammatory conditions but also for cancer.
Realistically, Amgen’s near-term outlook is not that groundbreaking. However, the overall valuation and potential of its M&A dealmaking are compelling enough to encourage investors patient enough to wait for the rewards in the long run.