“Anything that can go wrong will go wrong.”
It looks like Murphy’s law is about to strike again this year. The number of COVID-19 cases has reached almost 15 million worldwide, with about 4 million found in the US alone. However, the pandemic isn’t showing signs of slowing down.
Now, another deadly virus described to manifest “all the essential hallmarks of a candidate pandemic virus” has been found.
Earlier this month, a team of scientists revealed that there’s a newly discovered influenza strain, which could be a variation of the H1N1 swine flu—the same virus that triggered a global pandemic back in 2009.
That health crisis infected roughly 61 million Americans and more than 700 million people across the globe.
Although there’s still no conclusive evidence, this H1N1 influenza strain also traces its origins in China.
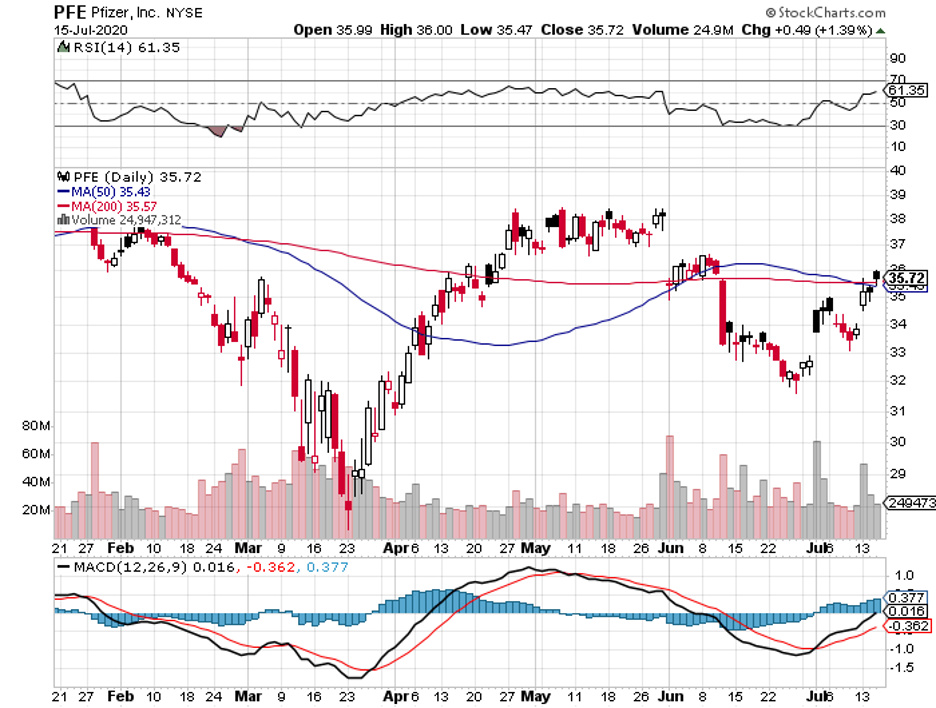
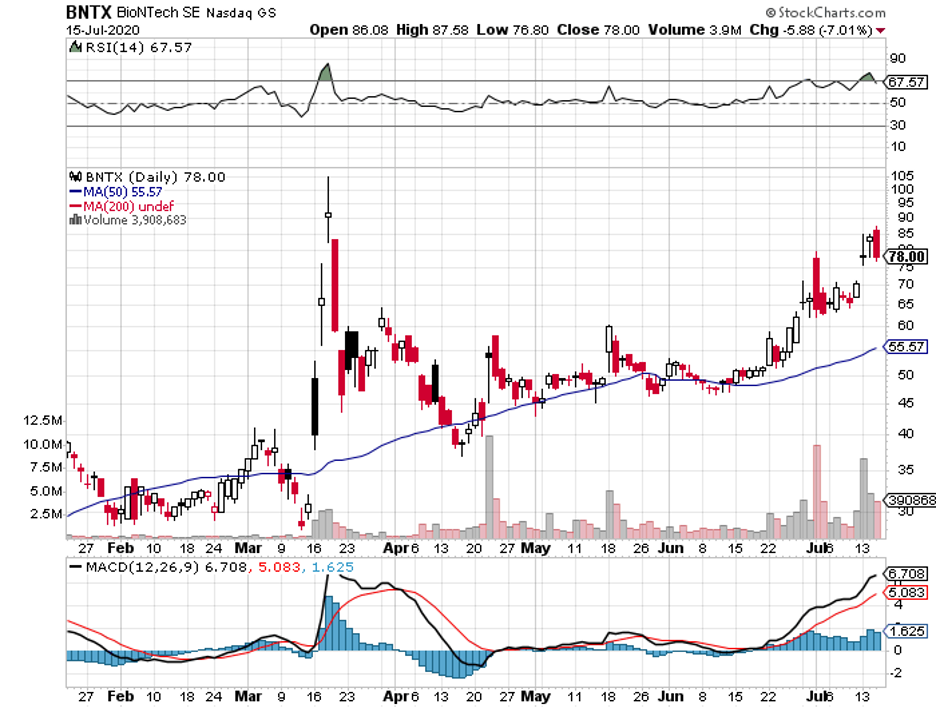
We witnessed how the stock market plummeted as the COVID-19 pandemic broke out. It eventually bounced back, which provided us with insights on how to deal with this potential second deadly virus.
Taking into consideration the uncertainty caused by these health and financial crises, I no longer put all my energy on near-term investments.
Instead, I train my eyes on stable and strong stocks with attractive revenue potential.
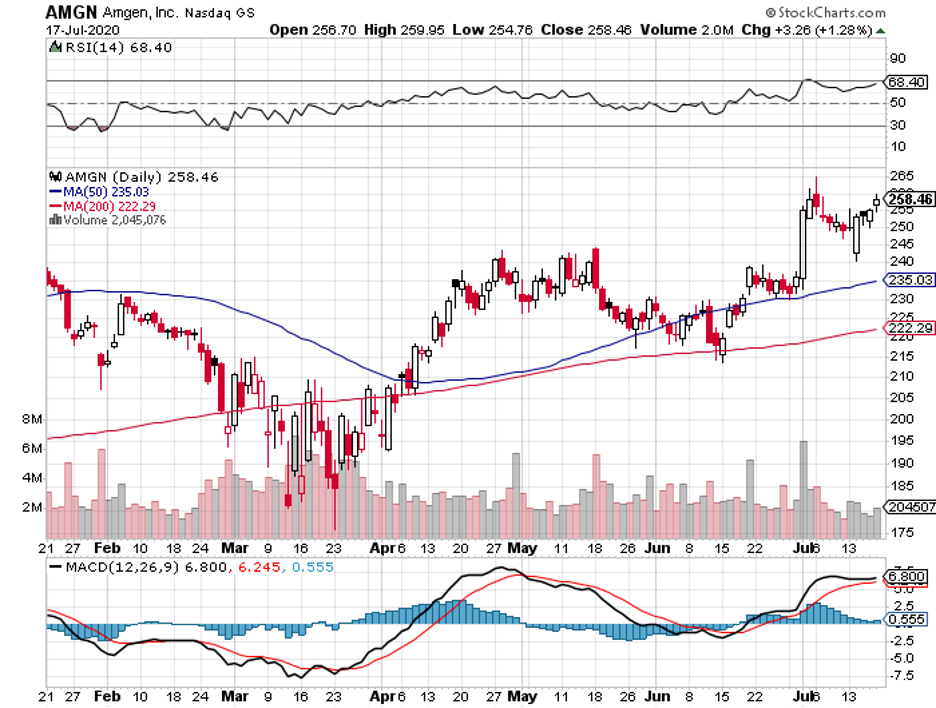
One of the companies that meet my criteria is Amgen (AMGN).
Amid the coronavirus pandemonium, Amgen has been aggressive in keeping its stronghold, particularly in its key moneymakers.
The latest win for the company is against Novartis (NVS), which challenged Amgen’s patent rights on the blockbuster anti-inflammatory treatment Enbrel.
This patent victory secured exclusivity for the top-selling rheumatoid arthritis injection, which generated $5.1 billion in sales in 2019 and could rake in at least $4.5 billion in 2020, against low-priced copycats until 2029.
Although Amgen has been struggling with biosimilar competition in the past years, the company’s first quarter earnings reports indicate that things are turning around for them.
Amgen reported an 11% year-over-year increase in revenue for the first quarter of 2020 to reach $6.2 billion, with global product sales jumping by 12%, boosted by a remarkable 15% in volume growth.
The company’s free cash flow for the first quarter also went up to $2 billion compared to the $1.7 billion it recorded in the same period in 2019.
The spike in Amgen’s numbers could be attributed to the new products in its pipeline. Apart from Enbrel, there are several other moneymakers generating solid growth for the company.
An obvious game-changer is severe plaque psoriasis medication Otezla, which Amgen acquired from Celgene for $13.4 billion in November 2019.
In the first 3 months of 2020 alone, Otezla has already raked in $479 million in sales for Amgen.
Sales of high cholesterol drug Repatha jumped by 62%, hitting $229 million.
Meanwhile, osteoporosis treatment Evenity contributed $100 million thanks to its expansion in the US and Japanese markets.
With the improvement in its performance, Amgen reiterated its revenue forecast for 2020 of $25 billion to $25.6 billion, showing off a 9.4% gain compared to 2019.
Aside from its current roster, Amgen is also waiting for regulatory approvals on some of its products this year.
The company is hoping for good news from the FDA on its multiple myeloma drug Kyprolis in November and its Rituxan biosimilar candidate in December.
Its pipeline also features 20 late-stage studies, 15 of which are for expanded indications of the company’s already-approved products.
Next to Otezla, Amgen is eyeing another blockbuster following the Fast Track designation granted to heart failure drug Omecamtiv mecarbil.
The drug, which the company is working in collaboration with Cytokinetics (CYTK), is projected to reach a jaw-dropping valuation of roughly $16 billion by 2026.
If successful, Omecamtiv mecarbil could become a close competitor of Entresto, which raked in $569 million for Novartis in the first quarter of 2020 alone.
Meanwhile, Amgen is not only focused on harnessing its growth drivers. The biotechnology giant has been active in searching for COVID-19 treatment as well.
Following the lead of Gilead Sciences (GILD), which used an already approved drug Remdesivir to come up with a treatment, Amgen is also testing its newly acquired blockbuster Otezla.
In using an anti-inflammatory drug to treat COVID-19 patients, Amgen is taking a similar approach as other biotechnology giants like Roche (RHHBY) with Actemra, Eli Lilly (LLY) with Baricitinib, and Sanofi (SNY) and Regeneron (REGN) with Kevzara.
Amgen investors currently get $1.60 in quarterly dividend payments, receiving $6.40 annually. In comparison, shareholders received $1.45 in 2019, showing off a healthy 10% hike.
With a stock price of roughly $235, this puts the company’s dividend yield to somewhere above 2.7%.
This is better than the 2% of investors earn on average from the S&P 500, indicating that Amgen pays investors with an above-average yield. Over the past 5 years, Amgen has boosted its annual dividend by nearly 103%.
Overall, Amgen is a solid long-term investment with promising growth drivers out in the market and in its pipeline.