If the volatility of the stock market lately has been making you sick, then it might be time to add more biotechnology and healthcare stocks to your portfolio. They’re all having the effect of dragging each other up in this superheated environment.
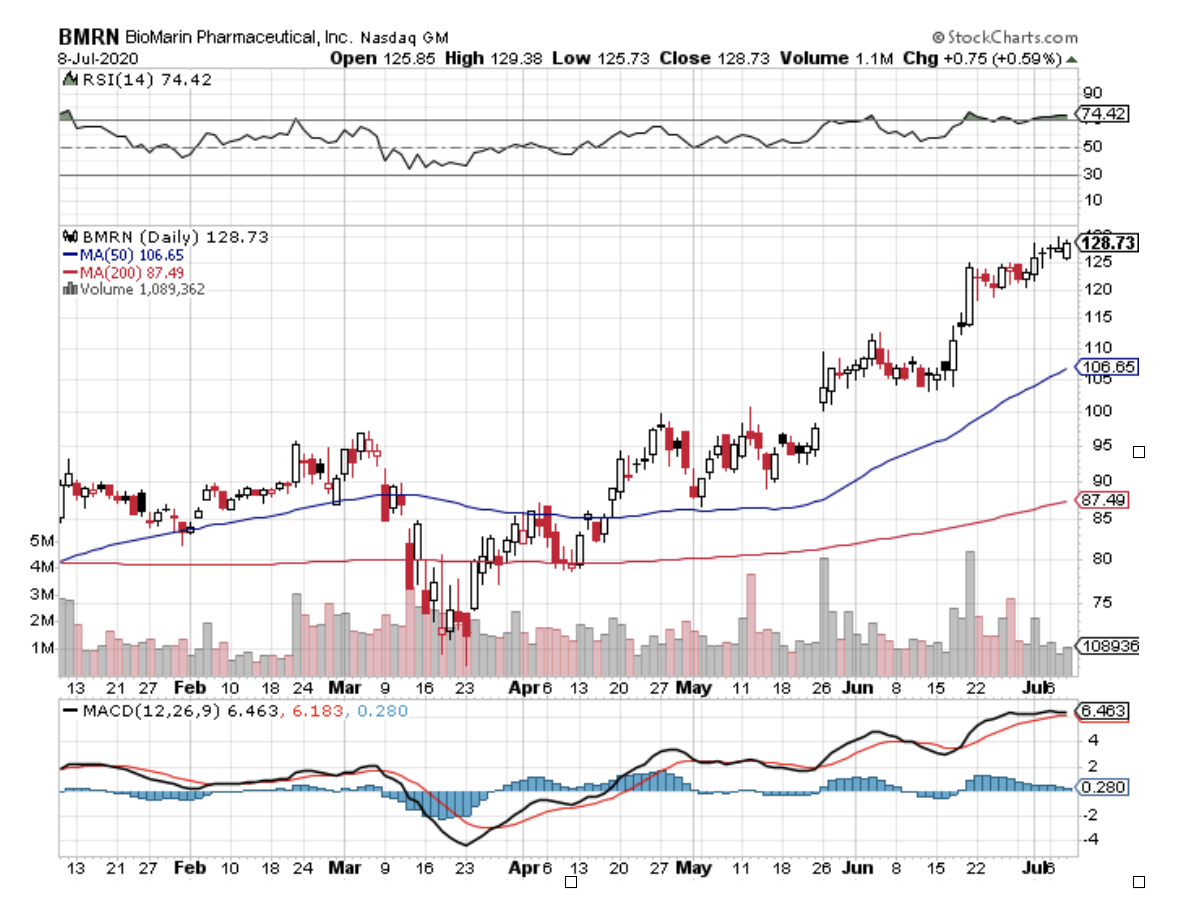
Investors looking for these businesses can find a safe haven in rare diseases big player BioMarin Pharmaceutical (BMRN), which has $16.5 billion in market capitalization.
While most businesses languished since the pandemic broke this 2020, this biotechnology company’s shares rose by 12% this year. That’s not where it ends, though.
BioMarin could still pick up even more momentum despite the second wave of COVID-19.
The company currently has an extensive pipeline aimed at rare genetic diseases, with seven already approved and marketed in over 75 countries across the globe.
Looking at BioMarin’s first quarter earnings report for 2020, the company recorded a revenue of $502.1 million. This shows a 25% increase from the $400.7 million it raked in during the same period in 2019. It also handily beat the analysts’ estimate of $468.8 million.
Among BioMarin’s products, genetic metabolic disorder infusion Naglazyme is considered the top-selling treatment.
Sales of this product climbed 32% year over year in the first quarter to hit $27.4 million, with the jump primarily attributed to an increase in sales in Russia and Brazil.
However, the rising stars of BioMarin are two enzyme replacement treatments.
One is genetic blood disorder treatment Palynziq, which skyrocketed by 181% year over year to reach $22.3 million.
The other is Batten disease treatment Brineura, which soared by a whopping 97% to rake in $24 million.
Three of BioMarin’s drugs also performed well during this quarter.
Phenylketonuria drug Kuvan recorded $15.1 million in sales, up by 14% from the same period in 2019.
Meanwhile, Morquio A syndrome medication Vimizim generated $11.4 million in sales, showing off a 9% year over year jump as well. As for Aldurazyme, this Hurler syndrome drug’s sales rose by 23% to reach 10.4 million.
Now, the company is looking into another rare-disease treatment, which could cover Hemophilia A therapies via its experimental gene therapy Roctavian. This can open up a huge market for BioMarin, with over 20,000 Americans suffering from this disease.
Roctavian is expected to receive the FDA green light earlier than its August 21 decision date, possibly marking another blockbuster drug added to BioMarin’s pipeline.
Needless to say, the steady sales growth of these products served as the major driver of the company’s improving bottom line.
Over the past 10 years, the annual revenue of BioMarin has consistently climbed and beat analysts’ expectations -- a trend that’s likely to go on in the next decade as well.
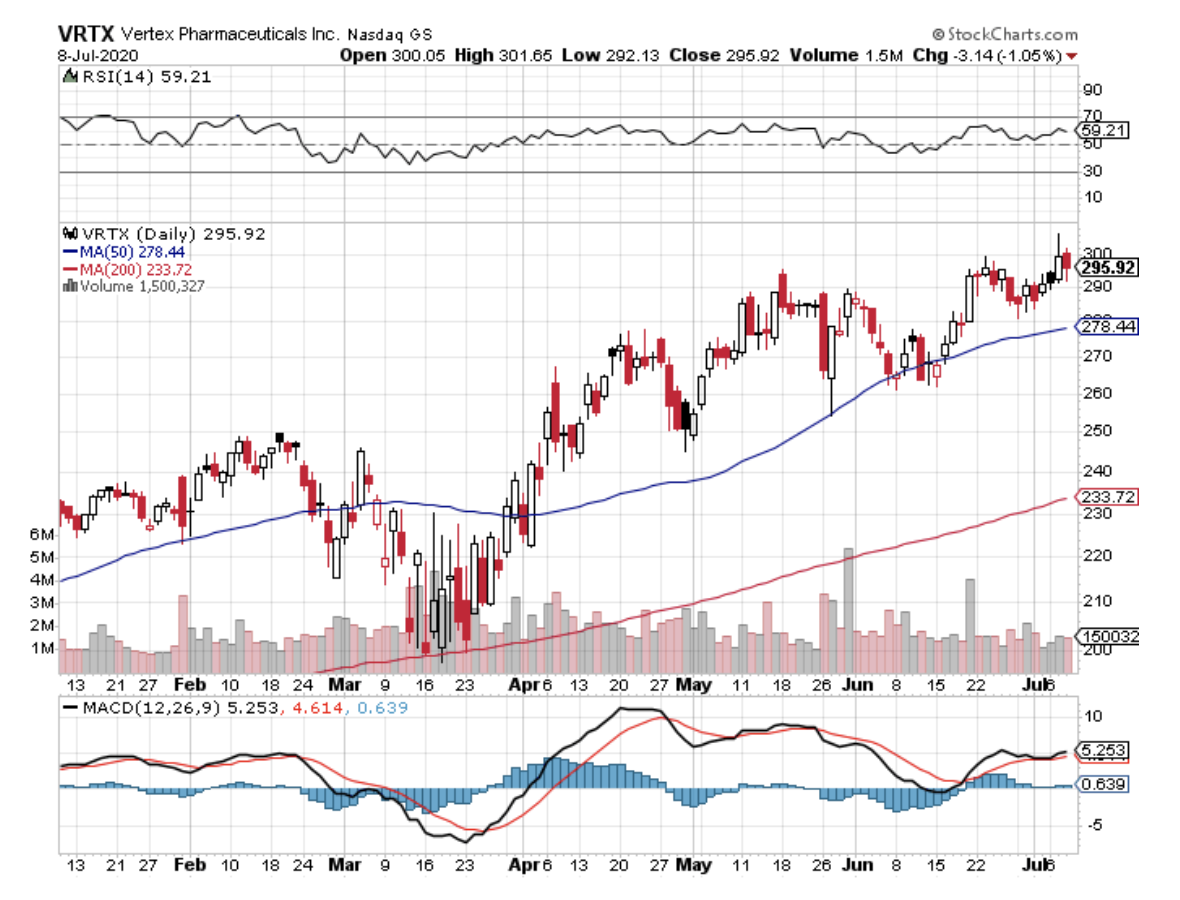
Another company that built its name on rare diseases treatments is Vertex Pharmaceuticals (VRTX).
While most stocks struggle to deal with black swan situations like the COVID-19 pandemic, Vertex is poised to enter the $100 billion market cap club soon.
In fact, the company recently updated its 2020 guidance, increasing its initial estimate from $5.1 billion and $5.3 billion to $5.3 billion to $5.6 billion.
In sum, Vertex is poised to continue its upward trajectory despite the current economic landscape.
The confidence in its growth is bolstered by its recent earnings report for the first quarter of 2020, which indicated an impressive 77% year over year to reach a net revenue of $1.5 billion.
At the moment, Vertex has $61.7 billion in market capitalization, with the company transforming into the most dominant player in the cystic fibrosis (CF) space. Actually, Vertex holds the monopoly on the approved drugs used to treat CF, namely, Kalydeco, Orkambi, and Symdeko.
Apart from its efforts to continuously dominate the CF sector, Vertex also has several moonshots that can eventually turn into major catalysts.
Among those is its partnership with CRISPR Therapeutics (CRSP).
The two biotechnology companies are developing a gene therapy called CTX001 which can cure rare genetic blood diseases. Specifically, CTX001 is designed to cure beta-thalassemia and sickle cell disease.
Apart from its partnership with CRISPR Therapeutics, Vertex also acquired Semma Therapeutics in 2019 with the goal of coming up with a cure for Type 1 diabetes. If things go as planned, a gene therapy for this genetic disease will advance to clinical testing by early 2021.
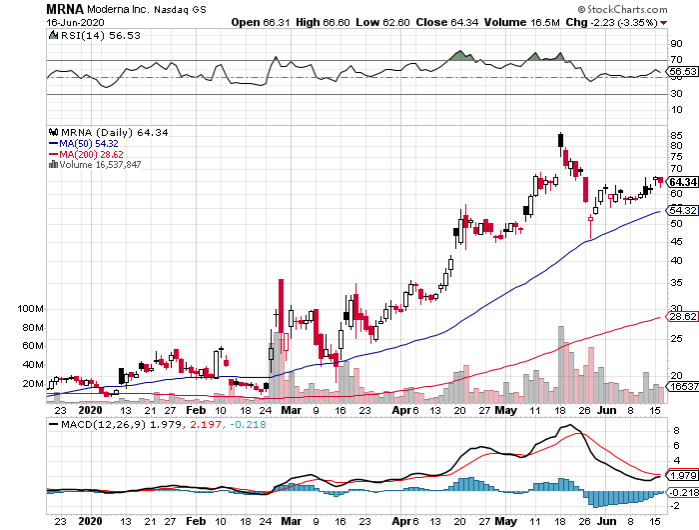
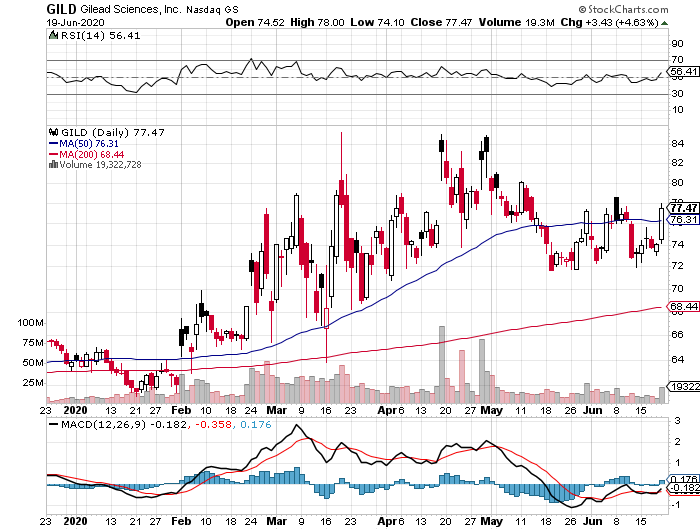
While the world is mired in crisis, the biotechnology sector has been fueled with excitement particularly because of the potential COVID-19 vaccines and cures from companies like Gilead Sciences (GILD) and Moderna (MRNA).
With insistent whispers that a second wave of the deadly COVID-19 is well on its way, opportunistic investors are actively seeking defensive stocks with businesses that can withstand the second wave of infections.
Both BioMarin and Vertex offer safe bets in this increasingly unpredictable world. Aside from proving their capacity to expand, both also have incredible room for growth.