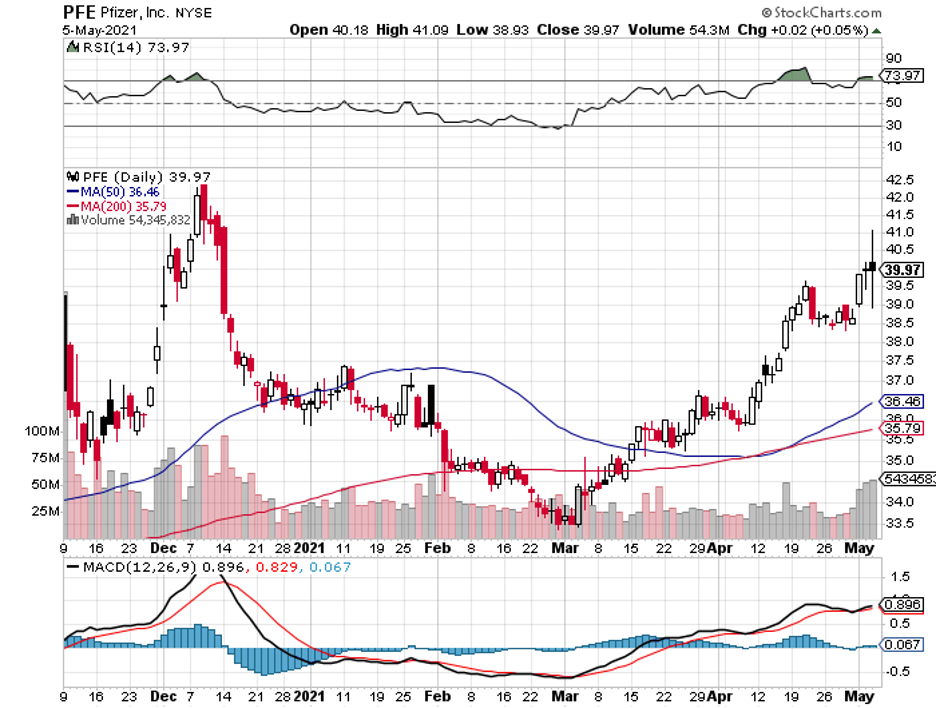
After a week of dissatisfying earnings reports from huge biopharmaceutical firms like Amgen (AMGN), Bristol-Myers Squibb (BMY), Eli Lilly (LLY), Gilead Sciences (GILD), and Merck (MRK), one company has managed to buck the trend: Pfizer (PFE).
In its first quarter earnings report for 2021, Pfizer reported adjusted diluted earnings of 93 cents per share, surpassing the earlier experts’ estimate of 77 cents.
Even its reported revenue exceeded the earlier predictions of $13.4 billion, raking in $14.6 billion during the period instead.
Aside from those, Pfizer also massively boosted its projected revenue from the COVID-19 vaccine it developed with BioNTech (BNTX).
Pfizer’s COVID-19 vaccine is slated for approval to be used for 12- to 15-year-olds by next week.
On top of these, the company expects data from its third COVID-19 vaccine candidate. This recent trial is for a booster dose, which could have results by early July and possibly a full emergency approval later on the same month.
The company now estimates $26 billion in sales for the vaccine, which is notably up from its $15 billion projection in February 2021.
Pfizer is also confident in its capacity to manufacture at least 3 billion doses of the COVID-19 vaccine in 2022, with the company already negotiating agreements with countries for their 2022 supply and beyond.
While the huge boost in the company’s COVID-19 vaccine sales expectations definitely grabs headlines, Pfizer’s base business brought in notable results as well.
Apart from the vaccine, the company’s operational growth in the first quarter was mostly driven by the sales from its blood clot treatment Eliquis, which went up by 25% operationally.
Sales of its heart drug Vyndagel soared by 88%, while its cancer drug Xeljanz jumped 18%.
One of the most notable moves from Pfizer is spinning off its off-patent drug division, Upjohn, to form a new company with generic drug developer Mylan, called Viatris (VTRS).
This decision would rid Pfizer of several well-known products, such as Viagra, Lyrica, Lipitor, Celebrex, and Chantix, which were responsible for roughly 15% of its total revenues.
However, sales for these items fell by 30% in the first nine months of 2020 alone—a chronically falling performance since 2017.
By eliminating the products that no longer hold any exclusivity rights and signing them off to Viatris, Pfizer can focus on developing and marketing new and innovative treatments.
So far, this strategy has started to bear fruit.
At the moment, Pfizer has several attractive assets in its pipeline. One of them is non-small cell lung cancer (NSCLC) treatment Lorbrena, which could become one of the highest-selling products in the oncology market.
Lorbrena is estimated to grow to over $40 billion each year by the mid-2020s.
At this point, the drug is in its registration phase and was granted a priority-review status. That means approval is on the horizon in the not-so-distant future.
Other potential blockbuster oncology assets include prostate cancer drug Xtandi, NSCLC treatment Bavencio, and breast cancer medication Ibrance.
All these are in late-stage trials, which means they should be available to market soon.
In total, Pfizer currently has at least 33 drugs queued in either Phase 3 trials or registration. The list includes vaccine candidates, immunology treatments, and, of course, oncology assets.
While Pfizer lost Upjohn in 2020, it gained a new partner in GlaxoSmithKline (GSK). The two companies decided to merge their consumer healthcare programs.
This made them the biggest provider of non-prescription drugs across the globe.
By shedding its sluggishly growing assets, Pfizer managed to develop its culture into one that concentrates on developing and marketing new and innovative products.
Additionally, the company’s current portfolio holds several growing products with the potential for expansion.
Given all these changes, Pfizer raised its financial guidance for 2021 as well.
For this year, the company now estimates adjusted diluted earnings to be valued between $3.55 and $3.65 per share compared to the previous range of $3.10 to $3.20 per share.
In terms of its full-year revenue, the company raised it from its estimate between $59.4 billion and $61.4 billion to $70.50 billion and $72.5 billion.
In terms of its projected revenue compound annual growth rate, Pfizer reconfirmed that it could deliver at least 6% through 2025 and a double-digit growth on its bottom line.
Remarkably, this is still not taking into consideration its COVID-19 vaccine.
If you pull out the revenues from its COVID-19 vaccine, then the company’s projected EPS growth for 2021 is at 15%.
Adding the vaccine into the equation gives us an impressive 41% increase in its EPS.
If you consider the wild card that is Pfizer’s COVID-19 vaccine, which would include a price increase coupled with the possibility of booster shots administered annually, and combine it with its base business, then it’s easy to see how the company’s growth could be turbocharged in the next few years.