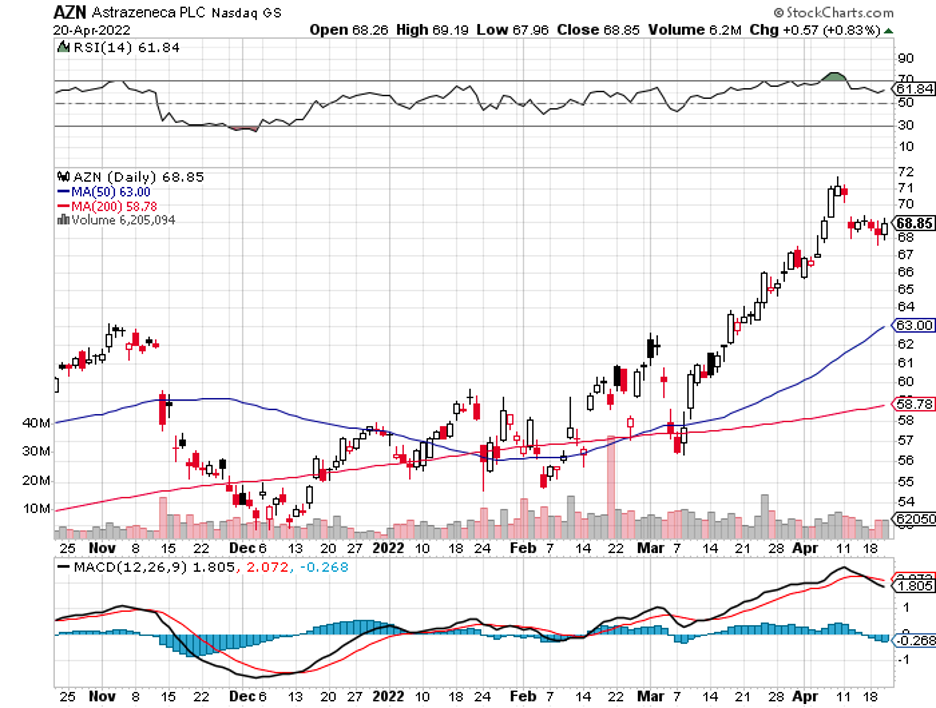
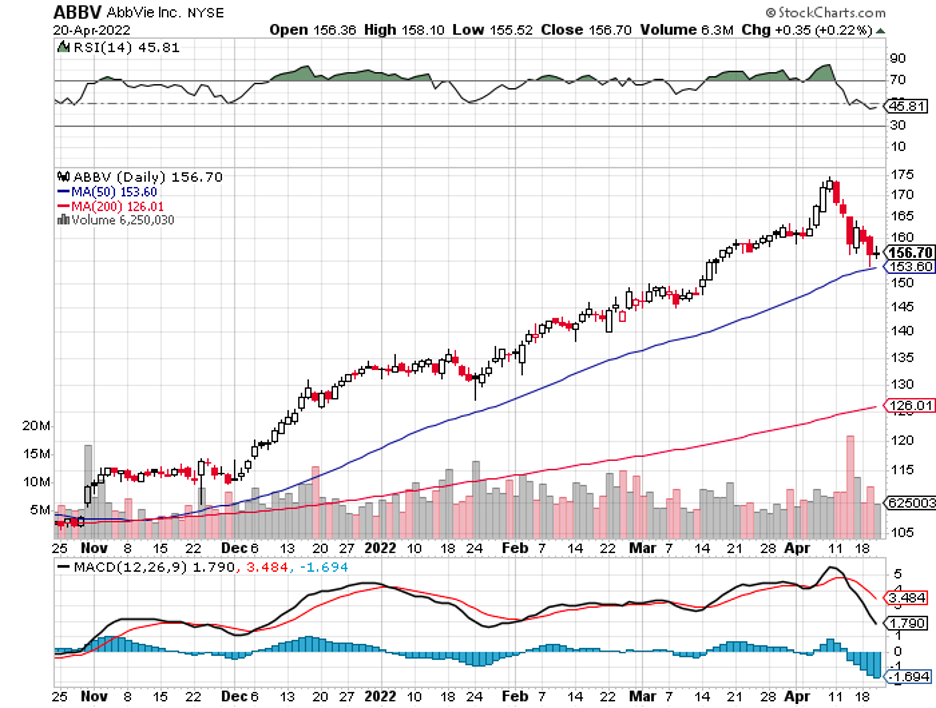
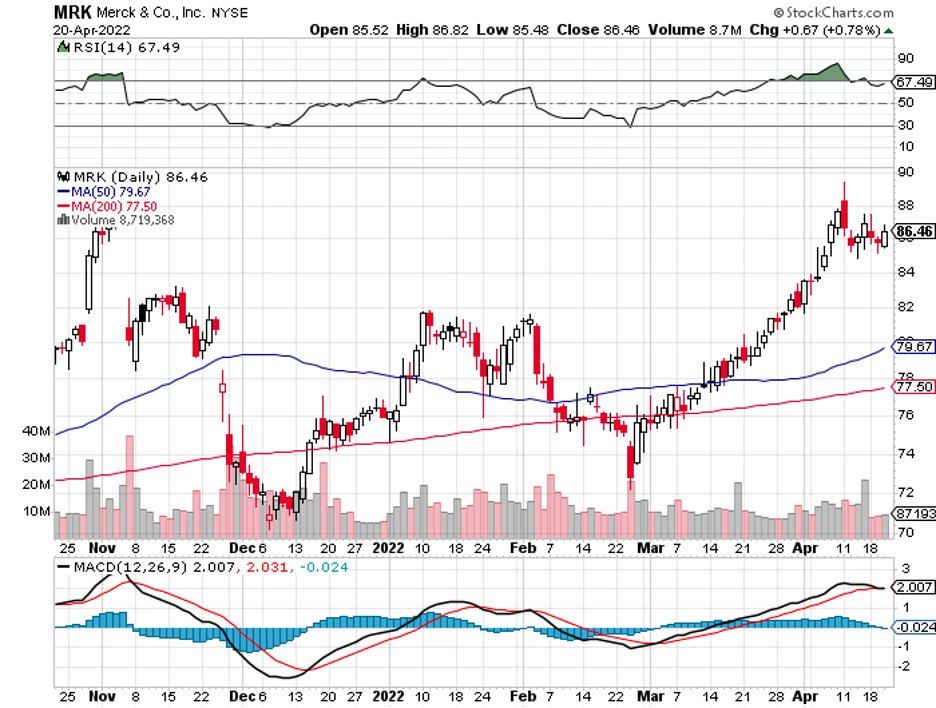
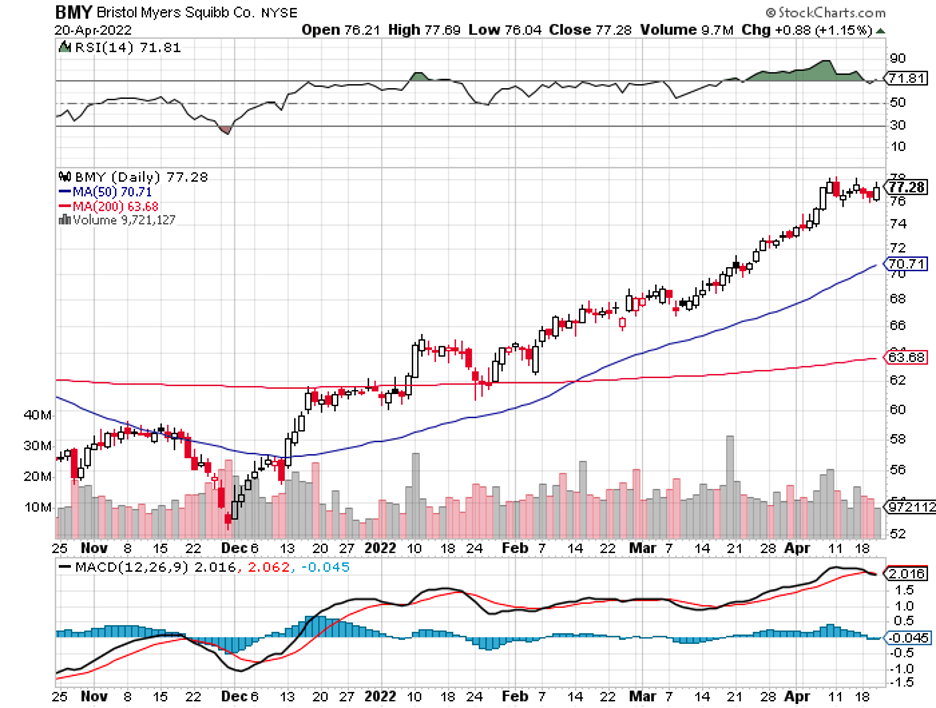
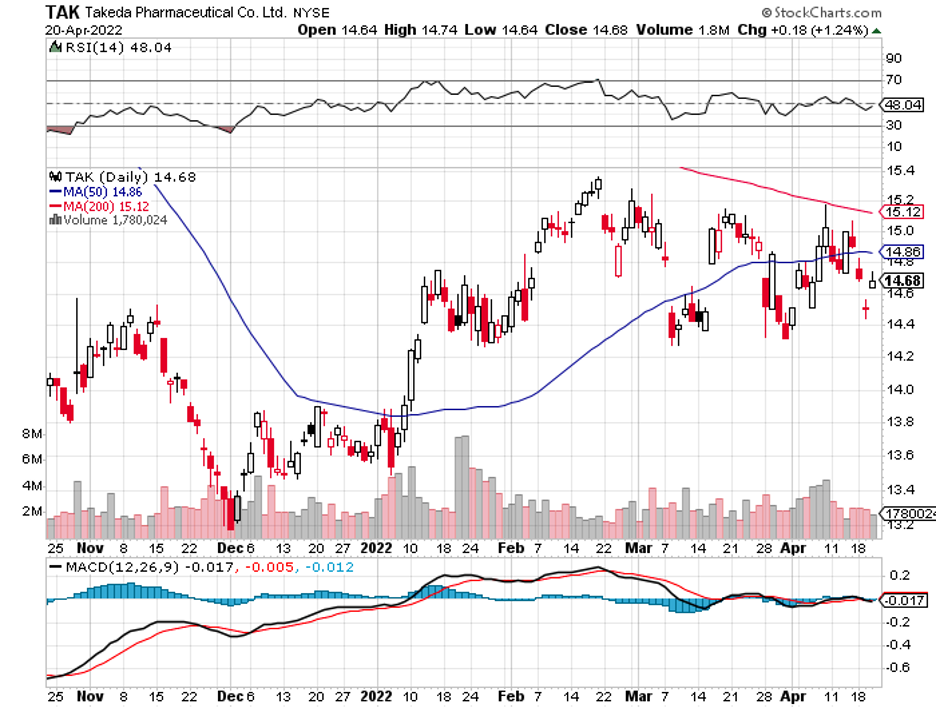
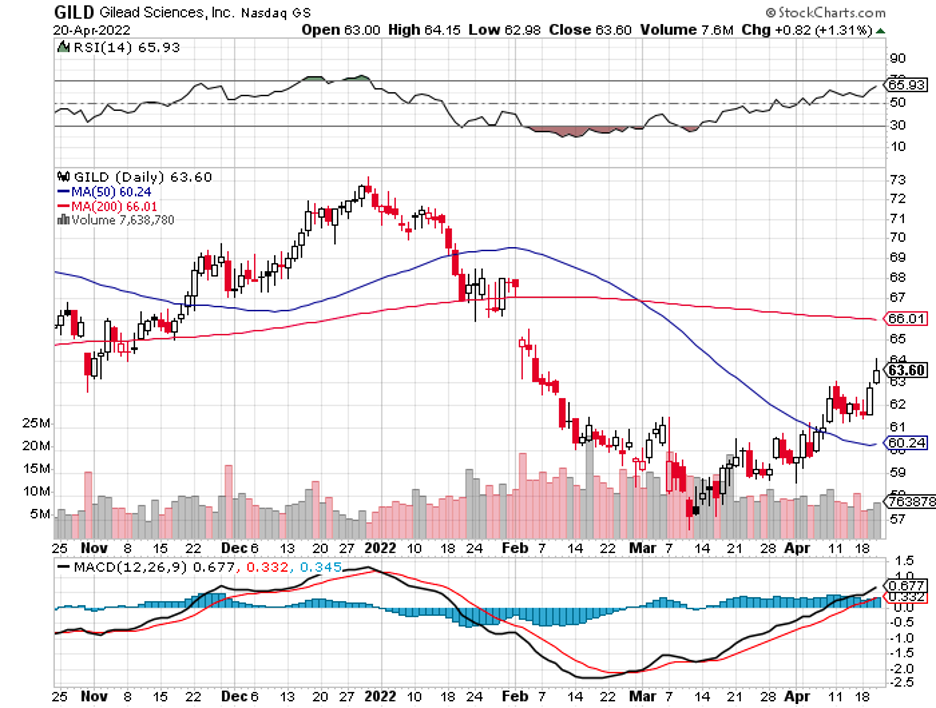
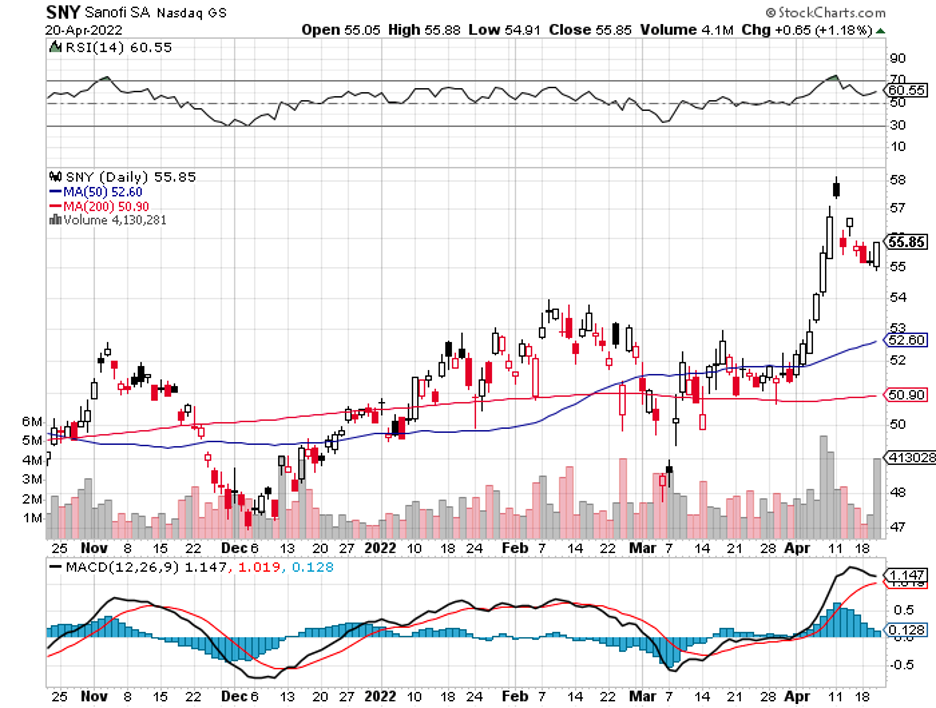
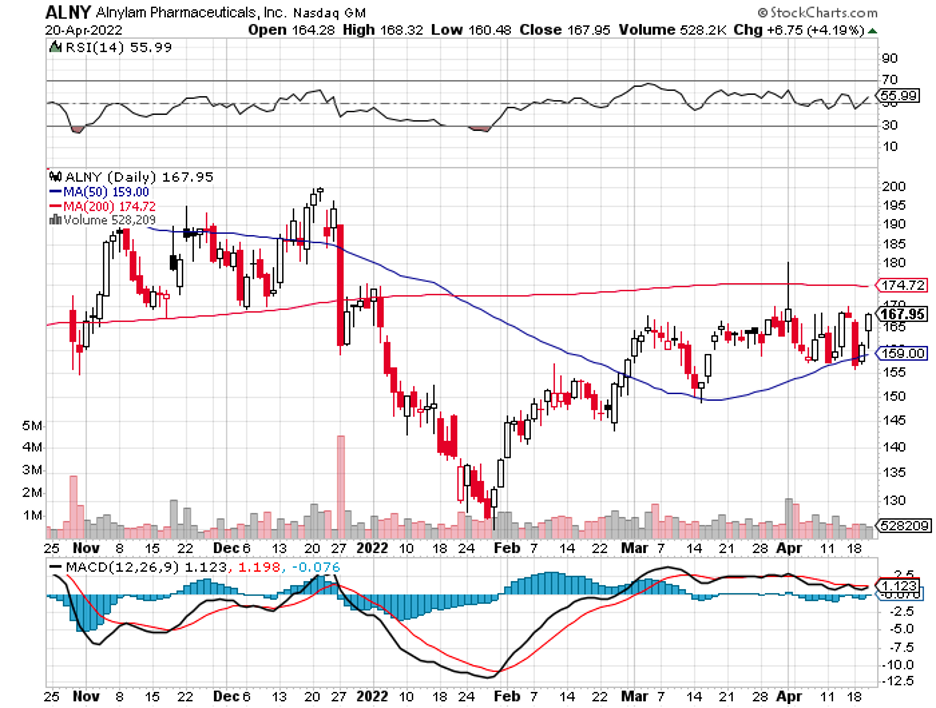
A stock with high margins offers a better buffer to face adversity and increased expenses.
Suppose a business’s revenue cost is substantial and leaves minimal room for the top line to maneuver and cover overhead and other operating fees. In that case, it can be challenging to stay in the black.
A recent example is Amazon (AMZN), which reports that its cost of sales typically comprises 80% or more of its revenue. When it struggled with the rising expenses in the past quarter, this e-commerce giant posted its first-ever loss in years.
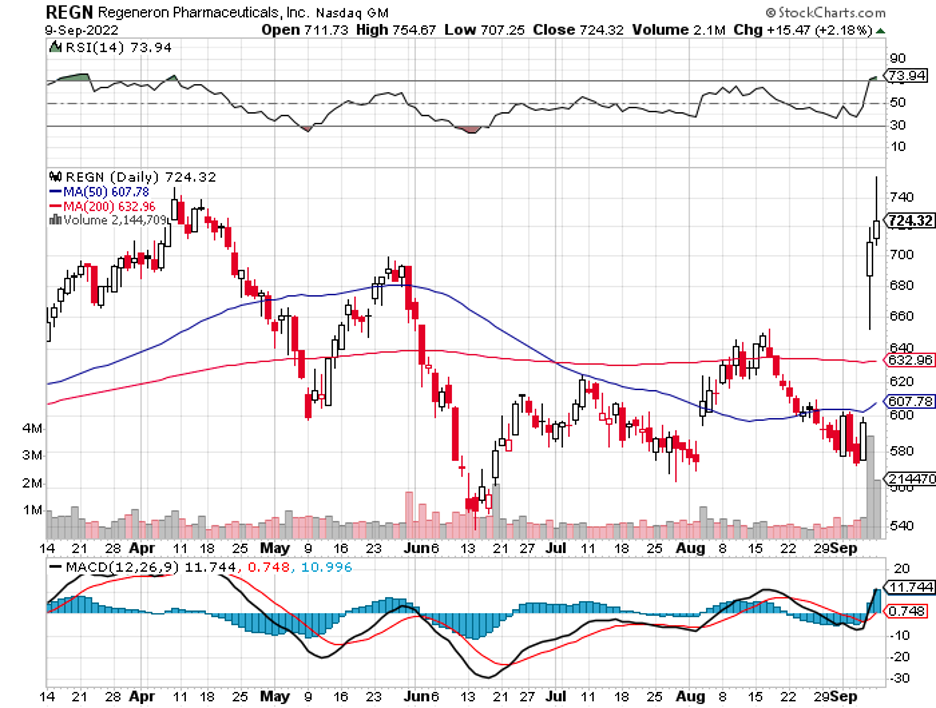
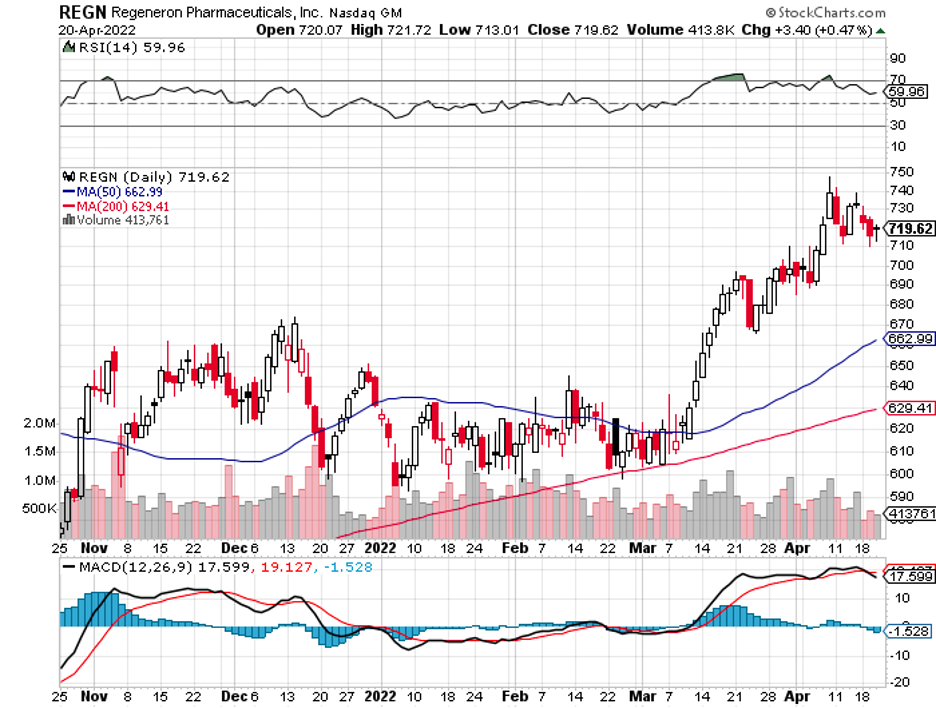
Only a handful of companies manage to stick with this principle. In the biotechnology and healthcare sector, one business that doesn’t have this issue and enjoys gross profit margins of at least 80% or better is Regeneron Pharmaceuticals (REGN).
Regeneron was recently spotlighted following a significant 19.2% jump in its price, translating to a more than $12 billion rise in market capitalization.
The catalyst for this double-digit climb is none other than Regeneron’s crown jewel: Eylea.
The anti-blindness treatment, jointly developed with Bayer (BAYRY), delivered excellent results in two late-stage trials.
This is a huge deal because it supports the application of Eylea at higher doses and longer-lasting intervals.
Previously, Eylea was only permitted to be administered in 2 mg every 8 weeks. The recent trial results proved that the medication can be given at a higher concentration of 8 mg in a more extended period of 16 weeks and can still be effective at battling the disease. Plus, it shows a similar safety profile as the currently approved dosage.
This is a timely development for Eylea, which is set to lose its patent exclusivity soon, bringing anxiety to shareholders.
For context, Eylea accounts for $3.13 billion of Regeneron’s sales in the first 6 months of 2022. In the year's second quarter alone, it generated $2.49 billion in sales, recording a 9% year-over-year increase in its global profits.
In 2021, this eye medication reported $9.4 billion in sales worldwide.
These recent developments are eyed as potential solutions to Eylea’s impending franchise exclusivity loss as it attempts to eliminate a key overhang.
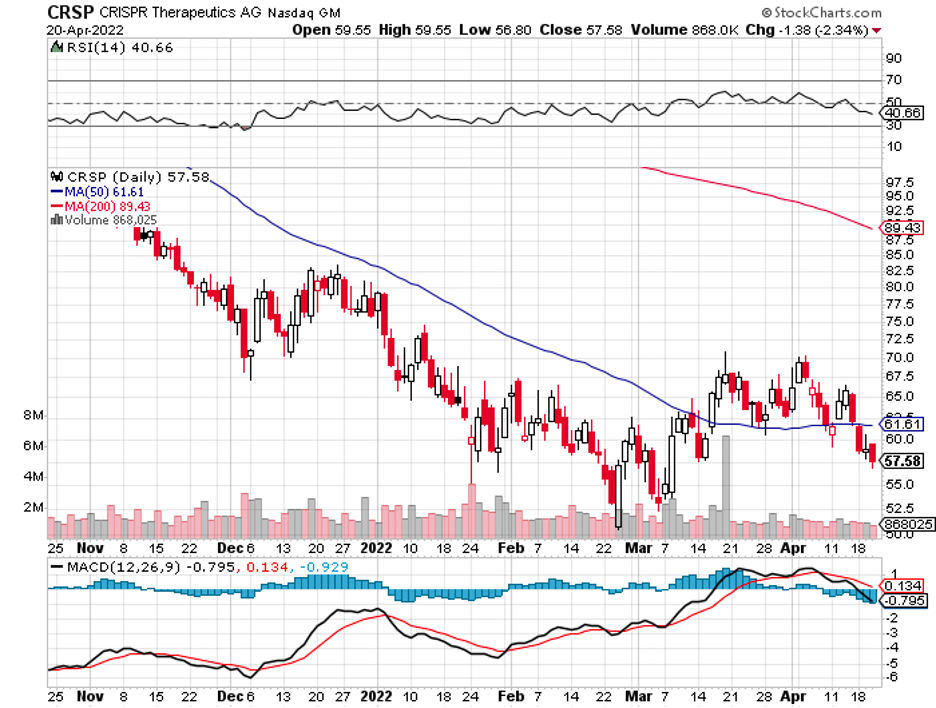
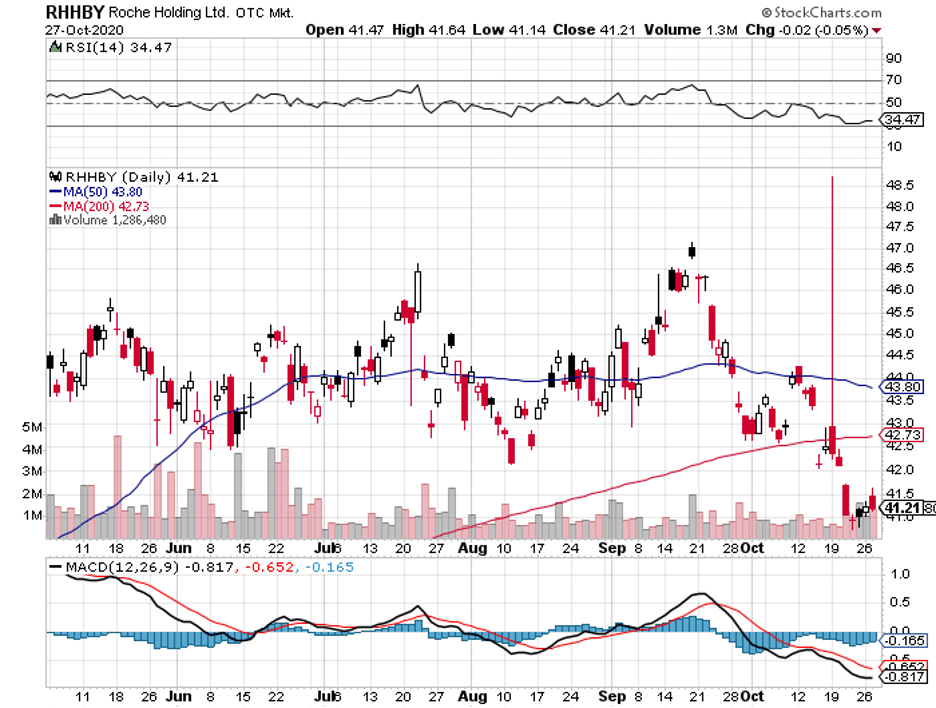
This move could slam the door shut on any talks or fears of potential copycats for at least a few years. It could also make it more competitive against rising competitors in the same space like Roche’s (RHHBY) Vabysmo.
The new data is expected to be used to defend the franchise from biosimilars, generic, and branded competitors. This is because patients are now offered an option for a treatment that needs fewer injections.
Most importantly, this could establish a firmer competitive moat for Regeneron and Bayer. After all, Eylea is projected to rake in more than $6 billion in sales in the US annually in 2023 and 2024.
Looking at their timeline and progress, the new Eylea dosage should be submitted for approval by the end of 2022 and launched by early 2023.
Other than Eylea, Regeneron has also been active in the oncology space.
Leveraging its roughly $6.2 billion sales from its COVID-19 treatment, Regeneron acquired several assets to expand its oncology segment.
Recently reported deals are its $900 million payment to Sanofi (SNY) to acquire non-small lung cancer drug Libtayo and the purchase of Checkmate Pharmaceuticals, which granted it access to promising melanoma candidates.
While these deals may not be as massive as other acquisitions in the industry, adding Libtayo and Checkmate Pharmaceuticals represent critical steps toward the right direction.
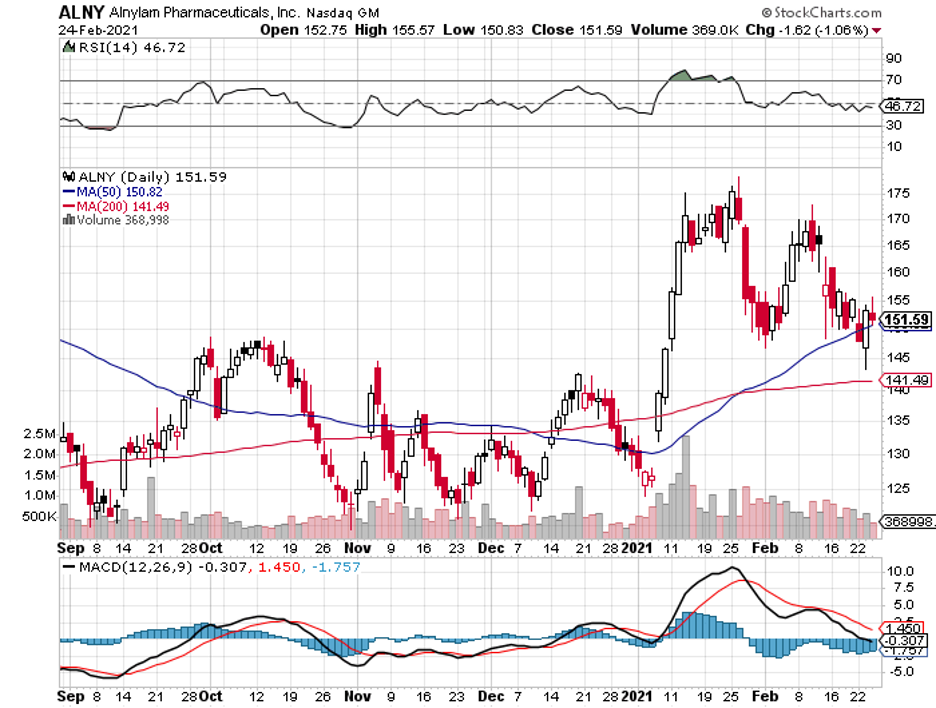
On top of these, Regeneron has existing partnerships and collaborations that would last for years. One is with Alnylam (ALNY), which involves treating liver cancer, ocular conditions, and diseases targeting the central nervous system.
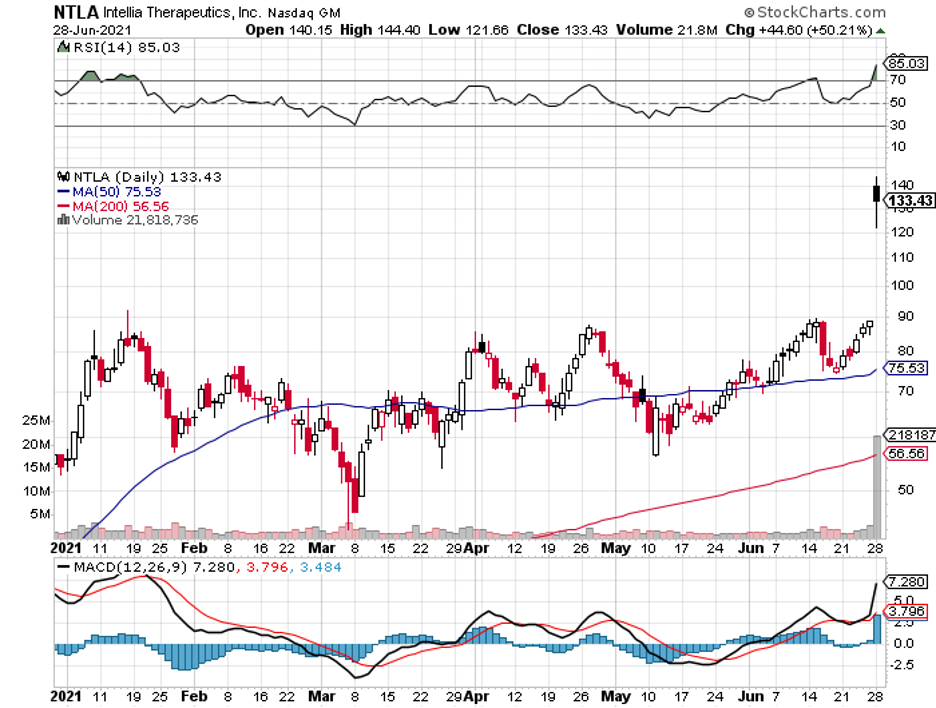
Meanwhile, Regeneron expanded its deal with Intellia Therapeutics (NTLA) to give more rights to their in vivo therapeutic candidate developed via CRISPR/Cas9 gene-editing technology and additional targets, particularly for hemophilia A and B.
Overall, Regeneron has been proving to be a noteworthy investment in biotechnology and healthcare. At this point, though, the recent clinical trial results have been added to its share price.
While it isn’t exactly cheap, it’s not an outlandish valuation either. In short, I suggest that you wait and buy the dip.