Tesla (TSLA) has been sizzling hot for months now, and it looks like its Midas touch has reached the biotechnology world.
It seems that almost everything linked to Tesla achieves success. That could indicate terrific news for a particular biotech: CureVac (CVAC).
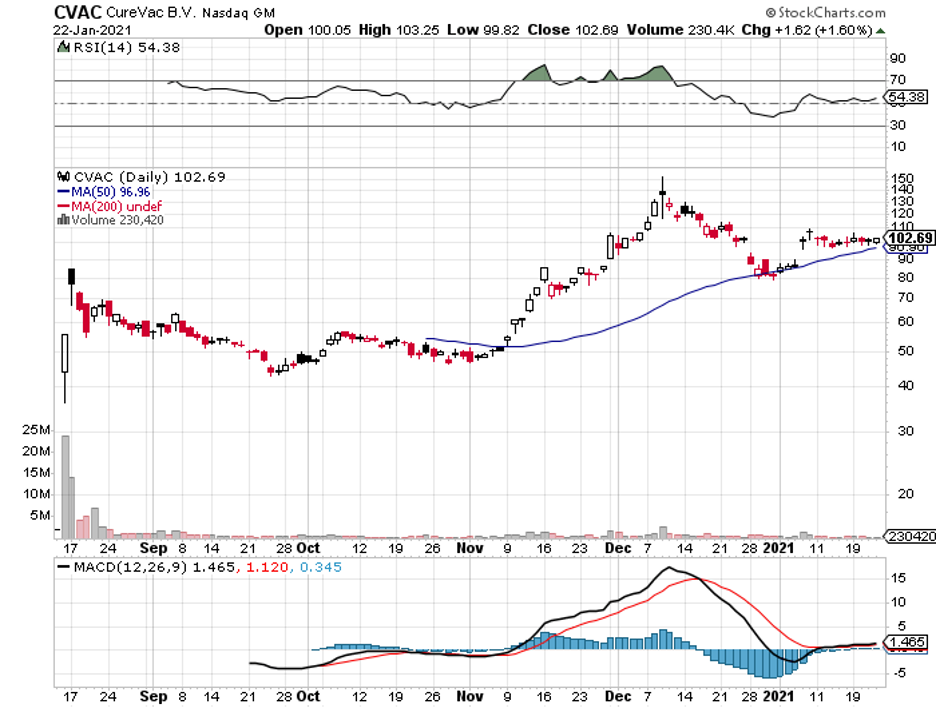
CureVac, an under-the-radar biotech stock, is closing in on the leading COVID-19 vaccine developers today.
A differentiating factor it has from the likes of Pfizer (PFE), BioNTech (BNTX), and Moderna (MRNA) is its bonafide tie-in with Tesla. Although it sounds like quite a stretch for an electric car company to have any involvement with a biotech stock, the connection actually makes sense.
Like Moderna and BioNTech, CureVac has also been working on utilizing messenger RNA (mRNA) technology to develop various vaccines and other treatments. If all goes well, this could even lead to finding a way to immunize people against cancer.
Where does Tesla come in?
It all started in 2019 when CureVac was awarded $34 million in funding by the Coalition for Epidemic Prepared Innovations (CEPI).
The goal was to create and eventually build a prototype of an mRNA “printer.” This high-tech tool would be used to produce mRNA doses in areas that suffer from viral outbreaks. It could be used by hospitals to create personalized medicines.
Having an mRNA printer would be groundbreaking in fighting off viral diseases, particularly in remote regions. As expected, this project faced many technology obstacles along the way.
Here’s where Tesla can offer a solution since one of the companies it acquired in the past years is Grohmann Engineering, which specializes in automated manufacturing.
This makes Tesla Grohmann Automation the logical partner for CureVac to turn for help in building its mRNA printer prototype.
What we know so far is that the two companies have been working closely on the project.
It’s only a matter of time before we find out if Tesla’s magic would once again blow our expectations out of the water and we are presented with yet another breakthrough.
Other than its alliance with Tesla in the mRNA race, CureVac has forged another partnership to transform itself into a stronger candidate in the COVID-19 vaccine competition.
CureVac has tapped into the global reach of Bayer (BAYN) to help it distribute its vaccine once it gains approval.
In terms of its own COVID-19 vaccine candidate, CureVac is anticipated to release positive results.
This is because its technology closely mirrors that used by Moderna and BioNTech, which strongly indicates that the efficacy levels could be just as good.
However, CureVac’s vaccine candidate offers a competitive advantage over the others: it doesn’t require cold storage.
This means it would be easier and more convenient to distribute it compared to Moderna’s and Pfizer’s.
It also requires a much smaller dose compared to Moderna’s COVID-19 vaccine candidate. This translates to cheaper manufacturing costs.
CureVac has secured a deal with the EU to deliver an initial 405 million doses for half of the year plus 300 million doses more in 2021 alone. It also agreed to produce 600 million doses in 2022.
Meanwhile, its alliance deal with Bayer indicates that it has secured a powerful distribution partner.
Therefore, we could expect CureVac to leverage Bayer’s global supply network to deliver its vaccines worldwide.
However, CureVac and Bayer are thinking way ahead of 2022.
The alliance formed by the two companies sees to it that the CureVac vaccine candidate would become the strongest contender in the post-pandemic years.
As per Bayer’s projection, the companies estimate 12 billion to 14 billion vaccine doses just to bring this pandemic under control.
Considering that COVID is expected to become an endemic disease, annual or even bi-annual vaccination programs would become the norm.
While Pfizer, Moderna, and AstraZeneca have been well ahead of the vaccine race, the door is still firmly open for other developers like Novavax (NVAX), Johnson & Johnson (JNJ), GlaxoSmithKline (GSK), Sanofi (SNY), and, of course, CureVac to launch their own COVID-19 vaccines.
Only going public in August 2020, this German biotech company already has $18.2 billion in market capitalization.
Its public offering of 15.3 million shares sold at $16 each generated $245.3 million for the company back in August.
By early December 2020, CureVac shares were already being traded somewhere around $150 as investors quickly began to realize the value proposition.
If I am to look to invest in a COVID-19 vaccine developer at this point, CureVac would surely be one of my choices.