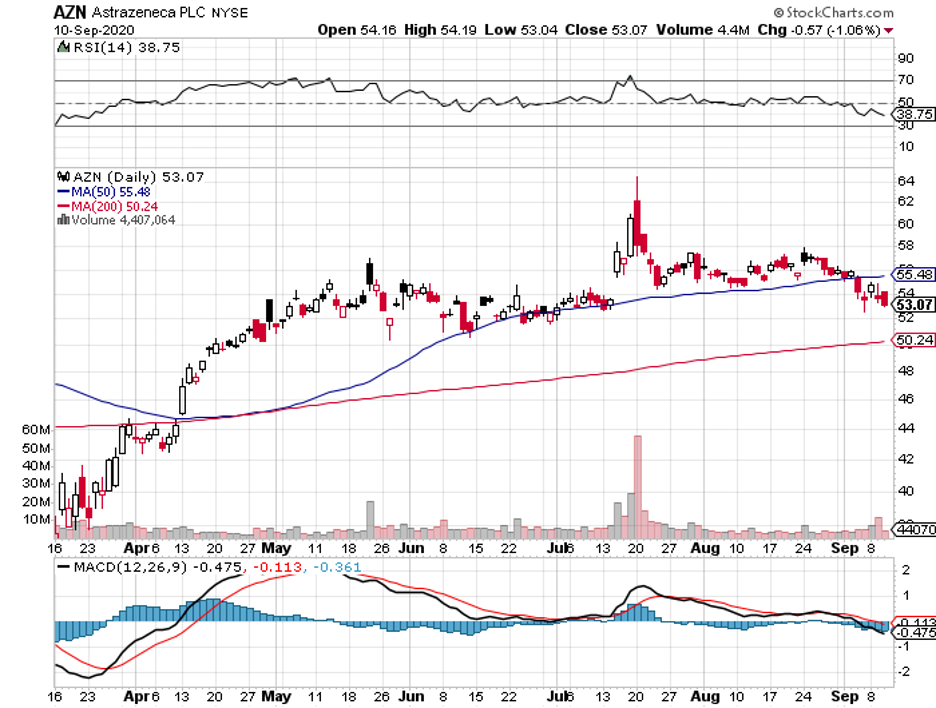
Vaccine developers have taken center stage on Wall Street since the pandemic started, with companies like Pfizer (PFE), Moderna (MRNA), and AstraZeneca (AZN) enjoying soaring share prices for months now.
One of the primary reasons for this popularity is the US government’s Operation Warp Speed, which poured $11 billion into its chosen COVID-19 vaccine programs.
Realistically, the cold, hard truth is that a COVID-19 vaccine will not be the panacea for this deadly virus.
While the vaccine developers are rushing to complete their clinical trials, more people continue to die from COVID-19.
With almost a million deaths and over 30 million cases recorded to date, the need for treatments is more pressing than ever.
Among the companies working on COVID-19 treatments, one name has been quietly making headway: Eli Lilly (LLY).
So far, the company has two potential treatments that can cure COVID-19 patients.
One is its rheumatoid arthritis drug Olumiant, which the company developed with biotechnology firm Incyte (INCY).
Results showed that the treatment can lessen the days patients stay in hospitals when combined with Gilead Sciences’ (GILD) Remdesivir. Not only that, the combination also reduced the severity of the disease and allowed for less-intensive hospital care.
Once all the results have been tested and validated, Eli Lilly will seek an emergency authorization from the FDA.
Aside from Eli Lilly and Gilead Sciences, Pfizer is also working on a potential COVID-19 treatment. Although not much is known about the New York biopharmaceutical giant’s version of the antiviral drug, the target approval date is set in the second half of 2021.
Riding on the momentum of its successful Olumiant trials, Eli Lilly is working to extend its winning streak by being one of the first to develop a preventive COVID-19 treatment specifically designed for elderly patients.
Eli Lilly is developing the potent monoclonal antibody treatment, called LY-CoV555, with AbCellera. The Phase 3 trials conducted in nursing homes were launched in August and the company expects the results to be available by March 2021.
While using monoclonal antibody treatment is groundbreaking technology, Eli Lilly is not alone in the field.
The company faces considerable competition from other healthcare giants like AstraZeneca and Regeneron (REGN).
Nonetheless, the antibody market is massive enough for sharing, with this market estimated to rake in as much as $10 billion annually.
Conservatively speaking and assuming that Eli Lilly fails to attract major market share, there’s still a decent chance that the sales of LY-CoV555 can go beyond $1 billion every year starting 2022.
Outside its COVID-19 programs, Eli Lilly is a dominant player in the diabetes market, with Trulicity leading the charge along with up and coming products like Humalog, Jardiance, Basaglar, and Humulin.
The company is expected to attract at least 13.8% of the market share this year, ranking second only to Novo Nordisk (NVO) and its 30.7% hold of the sector.
In the second quarter earnings report this year, Trulicity sales showed a 20% year-over-year jump to reach $1.2 billion in that period.
This is an impressive performance as investors expect the diabetes drug to surpass its 2019 sales of $4.1 billion.
Although Trulicity delivers substantial sales, it is remarkable that Eli Lilly is not overly reliant on the drug.
In fact, the diabetes drug’s total revenue only accounts for less than one-fifth of the company’s overall sales.
To boost its presence in the diabetes market, Eli Lilly added another potential blockbuster in its pipeline: Tirzepatide.
This drug is projected to become “best-in-class for lowering glucose, weight loss, and cardiovascular risk.”
To date, Tirzepatide is undergoing Phase 3 trials to test it on diabetes, obesity, and heart disease. It is also queued in Phase 2 trials for the liver disease NASH.
The potential of Tirzepatide is hinged not only in being a diabetes drug but more importantly, as an obesity drug.
If successful, Tirzepatide is estimated to hit peak sales of $10 billion annually, with the number trailing by 2025 to record $3.7 billion.
Another potential moneymaker for Eli Lilly is Verzenio, which showed an impressive 56% increase in sales in the second quarter to contribute $208.6 million.
In a bid to expand its oncology pipeline, Eli Lilly is looking into adding a new indication for Verzenio as well.
The company recently released the promising results for the oral tablet as an early-stage breast cancer treatment.
If successful, this drug will be in direct competition against an industry leader, Pfizer’s Ibrance.
In terms of its neurology pipeline, Eli Lilly has also been active in developing its own Alzheimer’s program.
While most of the treatments are still in the early stages, the success of Biogen’s (BIIB) Aducanumab could provide a much-needed boost for Eli Lilly’s own Alzheimer’s candidates.
Eli Lilly offers an extensive product line that goes beyond its COVID-19 programs, underscoring the company’s resilience even during the pandemic.
After dominating in the diabetes sector, the company focused its efforts on becoming one of the top players in the oncology, immunology, and neurology fields.
Consequently, Eli Lilly has been consistent in posting first-rate earnings and revenue growth since 2017.
Eli Lilly markets treatments for life-threatening and chronic conditions, with the company owning the rights to products with consistently growing sales. It also has the ability to continuously boost its revenue stream thanks to its rich pipeline and strategic collaborations.
The COVID-19 pandemic may have negatively affected sectors of Eli Lilly’s business this year, but the company holds the qualities that make it a solid long-term investment.