Patience is one of the key attributes that long-term investors need to cultivate, but practicing it is challenging. Stock markets are entirely unpredictable, and their ups and downs tend to rattle even the most experienced investors.
However, it’s essential to keep a calm mind and to be confident that the businesses you invest in have the fortitude to overcome even the most challenging economic or market downturn.
The biotechnology industry is an excellent place to search for stocks that can overcome market turmoils and succeed in the long run because the treatments they develop are so crucial to the lives of their clients.
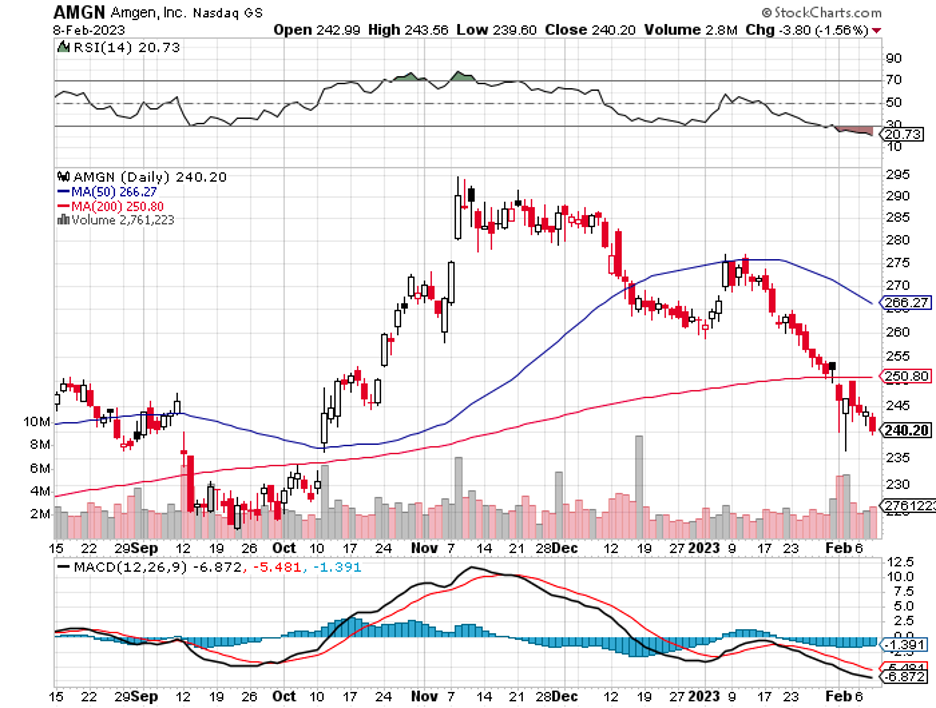
Amgen (AMGN) is a biotech that would make an excellent long-term investment.
This business, which has been a leader in the biotech sector since the 1980s, is among the largest in the world.
Amgen is also a member of the renowned Dow 30 companies, with a focus on oncology, biosimilars, and inflammatory diseases. In the past 10 years, it has established a strong track record and solid revenue growth trajectory.
The company recently released its fourth-quarter results, and they looked a tad flat on the surface. The report disclosed a total revenue growth of only 2%, which could have been caused by the pressures linked to pricing and competition around the company’s top-selling cholesterol-lowering treatment Repatha, migraine drug Aimovig, and immunology medications Otezla and Enbrel.
Still, Amgen continues to be a solid profit-making business, holding an A+ grade in terms of profitability. It sustains considerable pricing power on its treatments under exclusive patents and from its up-and-coming portfolio of biosimilar candidates.
Amgen has maintained a BBB+ rated balance sheet. It also pays a respectable dividend yield of 3.5%, with a well-protected payout ratio of 44%.
The company has also recorded consecutive growth in this aspect for 11 years. Looking at these figures, Amgen has scored primarily As in terms of consistency, growth, dividend, and yield.
Notably, the company’s foray into the biosimilar landscape would make long-term investors of the company quite happy soon.
Its long-awaited biosimilar version of the No. 1 selling drug worldwide, AbbVie’s (ABBV) Humira, has recently been launched to market.
Amgen’s version, called Amgevita, is the leading biosimilar in this market to date. It already has a five-month lead over the next competitor, arming it with a lot of time to establish a more competitive standing.
Beyond this candidate, Amgen has at least six more biosimilars that it plans to launch in the US and across the globe from 2023 until the end of 2030. This timeline would give the company excellent visibility in the long run.
Another potential biosimilar blockbuster is ABP 654, which is a biosimilar of Johnson & Johnson’s (JNJ) top-selling immunology treatment Stelara.
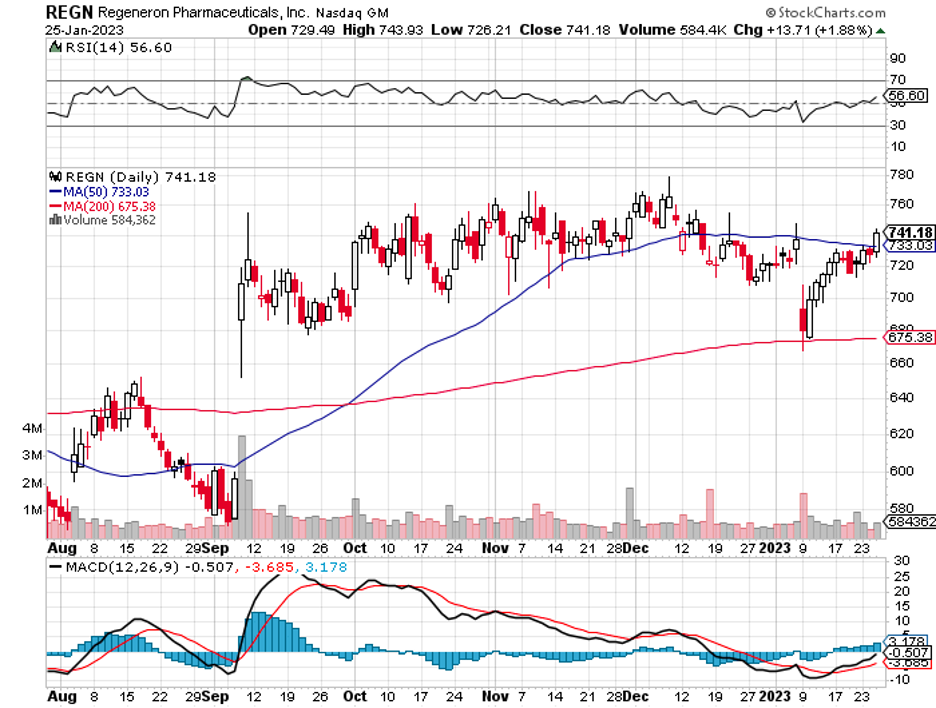
Amgen also has biosimilar versions of Bayer's (BAYG) and Regeneron’s (REGN) eye disorder drug Eylea and AstraZeneca’s (AZN) rare kidney disease treatment Soliris.
Basically, biosimilars are knock-offs for biologic drugs. They cost less because the manufacturers do not spend less in the research, trials, and approval stages. The processes are also shorter and less risky.
Biosimilars provide a way for patients and the whole healthcare system to save billions of dollars, signaling a bright future for this segment. They offer more affordable options to patients, which is an excellent response to the rising prices of medicines.
This means biosimilar development is far less speculative than creating a new drug, as manufacturers only need to replicate the already established results and success of the existing “original” drug.
Moreover, biosimilars bring with them a degree of pricing power. Unlike traditional treatments, no two biosimilars are allowed to carry the same biologic profile and should still undergo a stringent FDA assessment prior to gaining approval.
Overall, Amgen is a good option for long-term investors on the lookout for a quality biotech to add to their portfolios. Thanks to its burgeoning portfolio of potentially top-selling biosimilars, it has long-term solid revenue growth catalysts. These factors make Amgen a compelling buy on the drop.