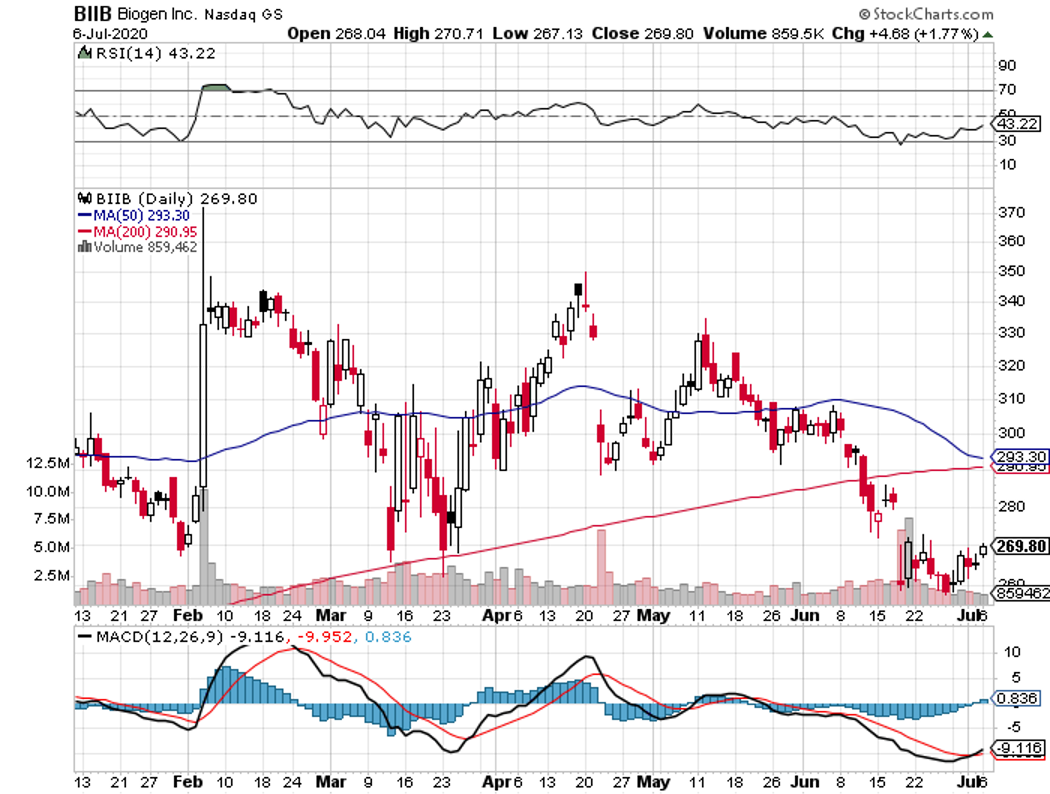
Biogen (BIIB) is a stock that perfectly fits the biotechnology mold.
Over the past 5 years, this Massachusetts-based company was down for roughly 25%. Five years before that though, Biogen stock catapulted 700%. A decade or so prior that, the company’s performance was flat, with a couple of extreme swings now and then.
However, the next decade could see Biogen stocks going upswing once again.
In the past 20 years, the biotechnology and healthcare sectors have been obsessed with finding a cure for cancer. With over 1.8 million fresh cases diagnosed every year, it’s understandable why the oncology space has received the most attention over the years.
Apart from cancer, companies have also made significant progress in other pressing issues like infectious diseases and cardiovascular disorders.
Now, a new market is starting to demand attention as well: the neuroscience field.
With all the demands for treatments for other diseases though, companies pulled R&D dollars away from the neuroscience budget and poured those into less risky efforts.
In comparison, Biogen doubled down spending on its neurology research.
In fact, the company has spent over $10 billion in this sector in the last 5 years. This amounts to roughly 5% of its annual market capitalization.
To bolster its neuroscience efforts, Biogen is investing in gene therapy as drivers of future growth.
Just last April, the company bought $225 million of Sangamo Therapeutics (SGMO) stock. On top of that, Biogen paid the smaller company $125 million for technology licensing. The deal also included up to $2.37 billion in royalties and milestone payments.
This newly established collaboration will see Sangamo working with Biogen to come up with gene therapies for various disorders, including Alzheimer’s disease and Parkinson’s disease.
At the moment, Biogen is focusing on the development of its Alzheimer’s treatment Aducanumab.
Alzheimer’s is a huge untapped market opportunity that presents a substantial unmet clinical demand. Right now, there are no approved treatments that could alter the natural progression of the disease.
In the US alone, there are more than 5.8 million people living with Alzheimer’s and about 500,000 new cases added annually.
This target makes Biogen’s Alzheimer’s treatment Aducanumab a potential mega-blockbuster.
Biogen’s estimated annual cost per patient for Aducanumab is $30,000.
With the number of Alzheimer’s patients in the US at the moment, back of the napkin math shows that Aducanumab can easily generate $15 billion in sales for Biogen.
Meanwhile, peak sales for this treatment could hit $20 billion — and this could even be an underestimate.
Projecting it further to 10 years down the line and putting Biogen’s market penetration at just 50%, assuming that the number of cases remains flat, then Aducanumab could reach 2.9 million users.
This means an annual astronomical cost of $87 billion for the Alzheimer’s market.
Let’s say Biogen is eventually asked to lower the price for the treatment to be accommodated by Medicare.
We use just a third of the $30,000, which puts the Alzheimer’s treatment at $10,000 each year for every patient instead. This would still rake in an impressive $29 billion for Biogen -- and these numbers only cover the US.
If we assume that the demand from the rest of the world matches the US sales, then global demand for Aducanumab could generate over $60 billion in a year based on our $10,000 per patient each year estimate.
Going back to Biogen’s initial $30,000 projection, then annual sales would reach a jaw-dropping $180 billion.
Sticking to the $60 billion per year estimate, Biogen can easily climb to $250 billion in market capitalization in the next 10 years --- an incredible jump from the $42.79 billion it has right now. The company’s shares could trade north of $1,500, providing its investors with over 400% return.
As a Roche (RRHBY) leader aptly described, “neuroscience has the potential to be in the ‘20s what oncology has been in the last decade.”
Now, Biogen is the undisputed leader in terms of pipeline candidates for the field. It has transformed itself into a research powerhouse in anticipation of its dominance in what could be the most important medical breakthroughs over the next decade or two.
After all, scientific breakthroughs allow us to live longer. In effect, a good part of our population will eventually face neurological problems that crop up later in life.
Hence, Biogen is poised to lead the charge in this grossly underserved market. The fact that the company has been keeping its pedal to the metal in terms of its R&D efforts further all but guarantees its dominance in the years to come.