“The moment you think you have the key to the market, they change the locks,” says an old Wall Street proverb.
When John identifies a strategic exit point, he will send you an alert with specific trade information as to what security to sell, when to sell it, and at what price. Most often, it will be to TAKE PROFITS, but, on rare occasions, it will be to exercise a STOP LOSS at a predetermined price to adhere to strict risk management discipline. Read more
When John identifies a strategic exit point, he will send you an alert with specific trade information as to what security to sell, when to sell it, and at what price. Most often, it will be to TAKE PROFITS, but, on rare occasions, it will be to exercise a STOP LOSS at a predetermined price to adhere to strict risk management discipline. Read more
Mad Hedge Biotech & Healthcare Letter
March 18, 2021
Fiat Lux
FEATURED TRADE:
(A BLUE CHIP STOCK SELLING AT A DISCOUNT)
(LLY), (GILD), (REGN), (SNY), (AMGN), (TEVA), (NVO), (ABBV), (BMY)
It’s not unheard of in the biotechnology industry to watch the stock prices of small or even mid-cap drug developers rise and fall by 30% following trial results or new drug approval.
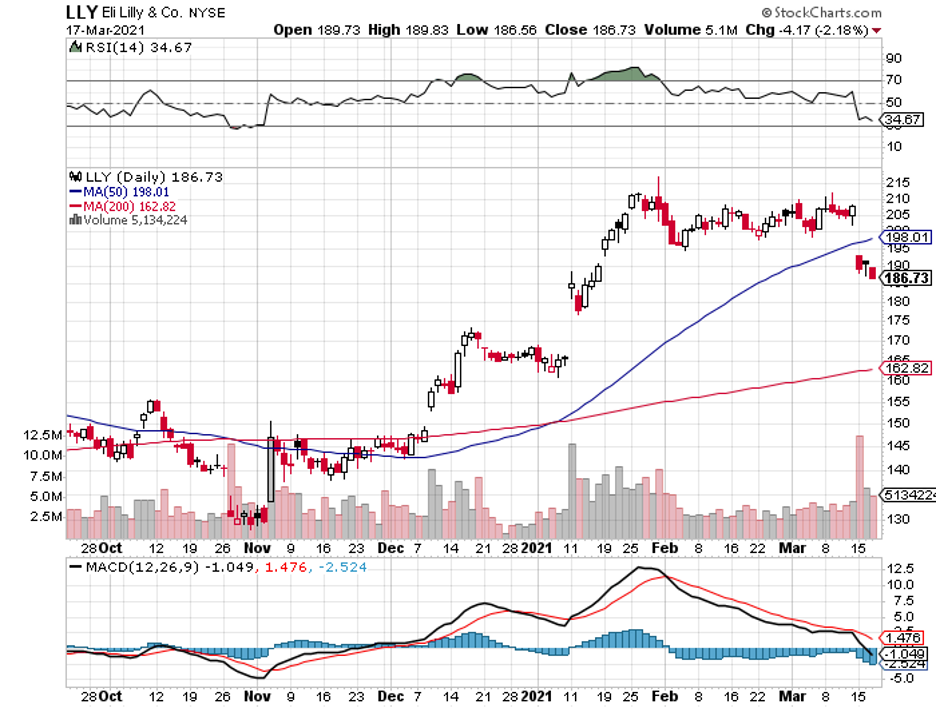
However, when the company is Eli Lilly (LLY), which holds a $179 billion market capitalization, then biotech investors need to pay attention.
After all, the only plausible conclusion to draw from this is that there have been some seismic advancements done by the company.
Two potentially breakthrough treatments are the culprit behind the volatility in Eli Lilly stock these days.
The first is Eli Lilly’s COVID-19 program, in which the company is looking into using Bamlanivimab (LY-CoV555) solo or combining it with Etesevimab (LY-CoV016).
What we know so far is that the combo drug can lower the risk of death and hospitalization among high-risk COVID-19 patients by as high as 87%.
In November 2020, the FDA granted Eli Lilly’s Bamlanivimab Emergency Use Authorization.
The solo treatment was also authorized for the same usage in Morocco, Europe, Canada, Rwanda, and some regions of the Middle East, where Eli Lilly is collaborating with the Bill and Melinda Gates Foundation for distribution.
Last February 2021, its combo treatment received the same approval.
To date, Eli Lilly has shipped roughly 1 million doses of Bamlanivimab and is committed to supplying an additional 1 million this quarter.
To meet the demand for the Bamlanivimab-Etesevimab combo, Eli Lilly will be working with pharmaceutical titan Amgen (AMGN).
In the company’s 2020 earnings report, Eli Lilly disclosed that Bamlanivimab accounted for $871 million of their sales.
For 2021, the market for COVID-19 treatments is valued at $27.25 billion.
Taking into consideration the competitors coming up with similar medications, such as Gilead Sciences (GILD), Regeneron (REGN), and Sanofi (SNY), the conservative estimate for the sales for Bamlanivimab alone is estimated to reach roughly $1 billion to $2 billion this year.
The second potential breakthrough that’s affecting Eli Lilly’s prices is its Alzheimer’s disease treatment, Donanemab.
Eli Lilly recently released positive data from the Phase 2 trial of Donanemab, with the treatment slowing down cognitive decline by 32% after 76 weeks.
In fact, a notable decline was already observed among the patients as early as 36 weeks.
This is an impressive result, and there’s talk that Eli Lilly’s plan of possible commercialization of Donanemab by 2024 could be fast-tracked to as early as the first half of 2023.
Interestingly, the positive news was met with negative reactions by the investors.
Eli Lilly fell by 9% following the Donanemab update, sending shares tumbling from $208.18 to $189.16.
This reaction effectively erased almost $20 billion in the company’s market value.
The negative reaction to Eli Lilly’s news may be stemming from the pending application of Biogen’s (BIIB) own Alzheimer’s drug, Aducanumab, which is expected to receive word from the FDA by June.
Investors anticipate that Aducanumab’s performance would be indicative of Donanemab’s future.
Looking at the trial results though, I can say that this shouldn’t be the case. Since the beginning, Donanemab has outperformed Aducanumab in practically every aspect.
Either way, what cannot be denied here is the market opportunity.
When the market thought that Aducanumab would get FDA approval in November 2020, the share price of Biogen saw a whopping 44% jump from $246 to $354 overnight.
Meanwhile, Donanemab’s potential sales volumes have been estimated to reach over $10 billion annually.
Other than Donanemab, Eli Lilly has been developing more contenders to boost its neuroscience division. Right now, this segment generates 6.3% of the company’s total revenues.
One of the promising drugs in the portfolio is migraine treatment Emgality, which recorded a 123% increase in sales last year to hit $362 million.
Thus far, Emgality holds at least 31% of the migraine market and still has room for growth and expansion.
This is a remarkable performance considering that its competitors include Amgen’s Aimovig and Teva’s (TEVA) Ajovy.
Another solid earner is antidepressant treatment Cymbalta, which generated over $768 million in sales last year, up by 5% year-on-year.
Outside its neuroscience efforts, one of Eli Lilly’s strongest growth drivers is its diabetes franchise.
This segment accounts for roughly 47% of its revenues and is led by Trulicity with $5 billion in sales last year, up 23% year-over-year.
Eli Lilly’s diabetes program has grown so much in the past years that it now aggressively competes against Novo Nordisk (NVO), a monopoly-like presence in this space.
In fact, Trulicity has been able to successfully protect its own market share against Novo’s heavily marketed Rybelsus, with data showing that users of Eli Lilly’s diabetes injectable recorded 60% adherence levels compared to Novo’s 43%.
In terms of expansion, Eli Lilly also won a new approval for Trulicity to be used to treat cardiovascular conditions as well.
This additional indication puts Trulicity’s peak sales at roughly $7.43 billion.
In an effort to corner the diabetes market, Eli Lilly also developed Tirzepatide.
Basically, this treatment is a long-term hedge against the pending loss of Trulicity’s patent exclusivity by 2027.
However, Tirzepatide is projected to surpass its predecessor in sales and reach double-digit billions.
Overall, Eli Lilly has positioned itself well in the diabetes market.
While it’s engaged in an aggressive battle for dominance against Novo Nordisk, there’s a lot of room for both.
The diabetes treatment segment is a continuously expanding market, with its value doubling in size from 2015 to 2015. Within this period, this market is projected to grow from $31 billion to $59 billion.
Aside from its diabetes and neuroscience programs, Eli Lilly has also been active in developing its immunology and oncology segments.
This is an ambitious plan, considering that practically all pharmaceutical companies are working on treatments in this space.
After all, the auto-immune market is massive as it’s worth well over $50 billion.
One of the bestsellers in Eli Lilly’s portfolio is plaque psoriasis treatment Taltz, which grew its sales by 31% year-over-year to reach $1.8 billion last year.
Some of the major competitors in this space are Bristol Myers Squibb (BMY) with Zeposia, Sanofi’s Dupixent, and AbbVie’s (ABBV) Skyrizi.
What could be promising news for Eli Lilly is the fact that AbbVie’s ultra-bestseller Humira is going off-patent by 2023.
This means that it could open up the market to allow both Taltz and Olumiant, another top-selling Eli Lilly treatment, to grab part of the lucrative market share.
Ultimately, Eli Lilly is a business that offers a promising commercialized portfolio and a remarkable near-term pipeline, which can reasonably support an annual revenue growth rate of roughly 10% even if we don’t factor in the effects of Donanemab.
Apart from the potential aftermath of the pending Biogen news, the fall in Eli Lilly’s shares could also be attributed to the extremely high expectation of investors.
Alzheimer’s has no approved cure, and there are only a handful of treatments developed from this neurological disease—none of which are even marginally effective.
It’s normal for investors to be wary of positive data results since they’ve been down this road before and are merely attempting to temper their excitement.
Amid the selloff, I believe that Donanemab is far from a lost cause. More importantly, I think the drop in Eli Lilly’s share price presents a rare buying opportunity for investors.
Therefore, I advise buying the dip.
When John identifies a strategic exit point, he will send you an alert with specific trade information as to what security to sell, when to sell it, and at what price. Most often, it will be to TAKE PROFITS, but, on rare occasions, it will be to exercise a STOP LOSS at a predetermined price to adhere to strict risk management discipline. Read more
When John identifies a strategic exit point, he will send you an alert with specific trade information as to what security to sell, when to sell it, and at what price. Most often, it will be to TAKE PROFITS, but, on rare occasions, it will be to exercise a STOP LOSS at a predetermined price to adhere to strict risk management discipline. Read more
Global Market Comments
March 18, 2021
Fiat Lux
Featured Trade:
(LEARNING THE ART OF RISK CONTROL)
Mad Hedge Technology Letter
March 17, 2021
Fiat Lux
Featured Trade:
(THE ANYWHERE ECONOMY)
(DOCU)
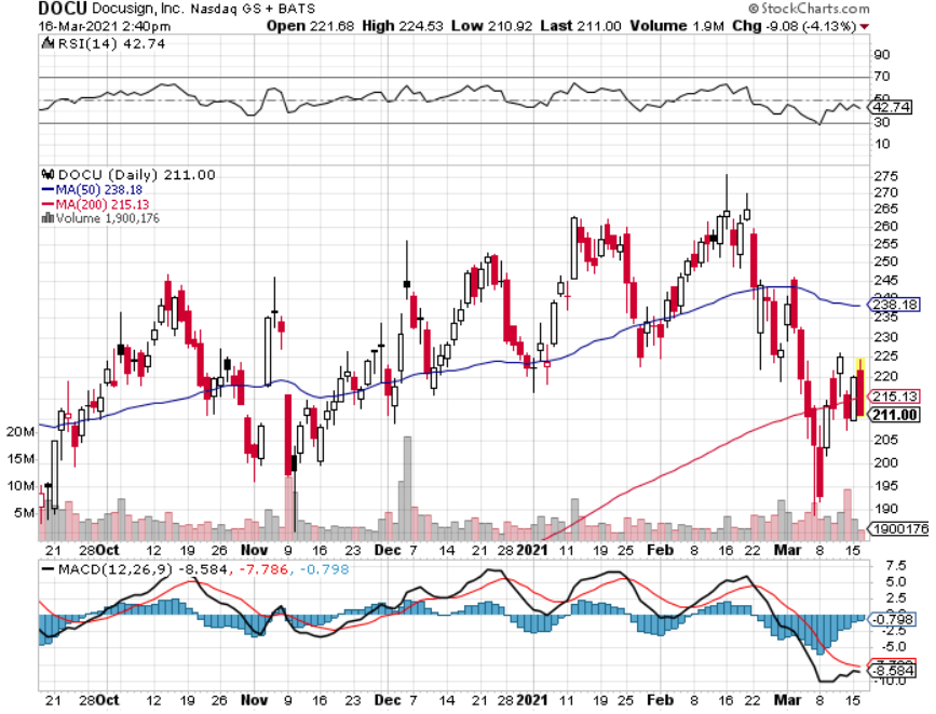
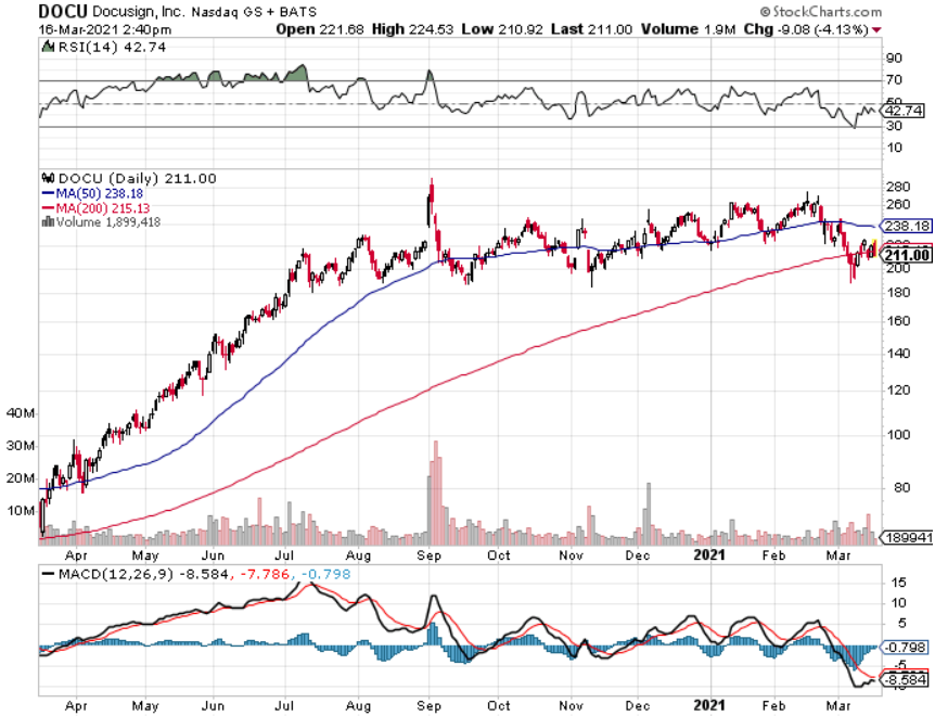
DocuSign (DOCU) is a U.S. cloud company providing e-signature solutions that enable businesses to digitally prepare, execute, and act on agreements and DocuSign’s CEO Dan Springer has described the current state of the economy by calling it the “anywhere economy.”
What does this mean?
Ultimately, now and over the coming years, he believes the trend will continue toward the option of doing anything from anywhere.
Springer has labelled the products and services supporting this trend the “anywhere economy”.
He believes the company he runs, DocuSign, is a critical piece to this anywhere economy, and it's only just beginning.
Springer wants investors to know that we are just in the first period of this hockey match and there’s a lot of ice time left.
The first period has been pretty good to DOCU and last year validated that by DOCU signing off 2020 nearly 50% bigger than they were in 2019 with almost $1.5 billion in revenue.
They gained new customers, expanded their existing relationship with others, and experienced a surge in adoption of DOCU products as accelerating digital trends already underway literally caught on fire.
The digital transformation of agreements is still fluid and progressing and this transformation not only allows agreements to be prepared, signed, act on, and managed from anywhere. It also allows greater speed and efficiency than manual paper-based processes.
Ultimately, DOCU's premium e-signing tools will force companies to never go back to paper even after the pandemic ameliorates.
DOCU also does not believe life will go back to the way it was before.
Of course, many in-person activities will be welcomed back.
But when consumers discovered optimal solutions during the pandemic, DOCU believes those will continue and flourish unfettered, whether it's total or partial work from home, virtual visits to medical professionals, or getting a document notarized remotely.
People aren't going back to paper.
They're not going back to manual processing.
What is the real question then?
The thing to ask now is whether the rate of new people coming to DOCU will change with the reopening of the society, economy, and the world?
This could possibly pull back the momentum in the demand environment, but DOCU has telegraphed to investors that a potential drawback would be temporary before the digital transformation reignites.
And let me get straight at this point, yes, DOCU hasn't seen a change yet and demand is following through greasing the revenue machine as we speak.
How is performance at DOCU?
Revenue growth of 57% and billings growth of 46% year over year.
DOCU onboarded more than 70,000 new customers last year, bringing the total to nearly 892,000 customers worldwide.
Their customers even displayed the robustness of their wallet with DOCU experiencing their strongest expansion and up-sell rates yet, driving their dollar net retention to a record 123%.
New customers were tripping over themselves to join DOCU with DOCU experiencing a customer addition rate more than double that of fiscal '20, edging them ever closer to the 1 million customer mark.
The use cases for DOCUs e-signature services are growing and it’s not just HR, procurement, customer service, and in-branch onboarding needs, there is way more left in the pipeline.
DOCU transactions took less than a minute to complete on average, delivering a rapid ROI.
And DocuSign went from a crisis response solution last year to a business-as-usual solution today.
One of DOCUs pipeline is international and last year just scratched the surface with international revenue increasing a head-turning 83% year over year to $89 million in the fourth quarter.
For the full year, international revenue grew over 67% to $287 million, reflecting accelerated expansion across geographies.
To give you a sense of the magnitude of DOCUs overperformance last year, they added nearly the same number of customers this past year as they had in total at the time they went public.
As part of that, DOCU added 11,000 new direct customers in Q4 for a total of over 50,000 for the year.
A first quarter guide follows much of the same rhetoric of explosive growth and DOCU expects total revenue of $432 million to $436 million in Q1 or growth of 45% to 47% year over year.
At the end of the day, all I hear from CIOs (Chief Investment Officers) are they've got a backlog of things they need to get done because the pandemic made it very difficult for them to get certain projects done.
And at the same time, they acknowledge their achievement last year couldn’t be possible without the blind digital transformation they undertook, and they want more of it with DOCU at its core.
So as I write this tech letter, I do predict another re-acceleration of the digital transformation story once the novelty of normalizing the world takes place.
And this normalization doesn’t even need to take place for a considerable cross and up-sell opportunity this year, and those incremental customers, significant customer new additions that drive DOCU's bottom line.
Many renewals will come up after the first year of DOCUs offerings, and I believe not only should it be a great cross-sell opportunity, but they will be happy to renew DOCU's products in full without question.
Long term, this is a great tech company to buy and hold, but the tech sector is near all-time highs and trying to digest higher interest rates and higher inflation expectations.
After we absorb this, the next move is up.
Legal Disclaimer
There is a very high degree of risk involved in trading. Past results are not indicative of future returns. MadHedgeFundTrader.com and all individuals affiliated with this site assume no responsibilities for your trading and investment results. The indicators, strategies, columns, articles and all other features are for educational purposes only and should not be construed as investment advice. Information for futures trading observations are obtained from sources believed to be reliable, but we do not warrant its completeness or accuracy, or warrant any results from the use of the information. Your use of the trading observations is entirely at your own risk and it is your sole responsibility to evaluate the accuracy, completeness and usefulness of the information. You must assess the risk of any trade with your broker and make your own independent decisions regarding any securities mentioned herein. Affiliates of MadHedgeFundTrader.com may have a position or effect transactions in the securities described herein (or options thereon) and/or otherwise employ trading strategies that may be consistent or inconsistent with the provided strategies.