Investing in quality stocks is a surefire way to slowly build a healthy portfolio over the years.
As long as you buy and hold stocks that have the potential to expand and offer stable financials continuously, then you’ll be securing your long-term success.
Obviously, this strategy is less risky compared to jumping on every new bandwagon and believing hyped ideas.
Then, there are breakthrough ideas that look too good to pass up. A good example is the cryptocurrencies such as Bitcoin and Ethereum.
Both operate on blockchain technology, which holds the potential to revolutionize practically all industries by decentralizing them and utilizing data in more efficient ways.
While investing in this kind of technology can definitely be exciting and thrilling, it’s undeniably scary for some who are still unfamiliar with it.
But, what if there’s a safer and more traditional way to get your foot in this groundbreaking technology?
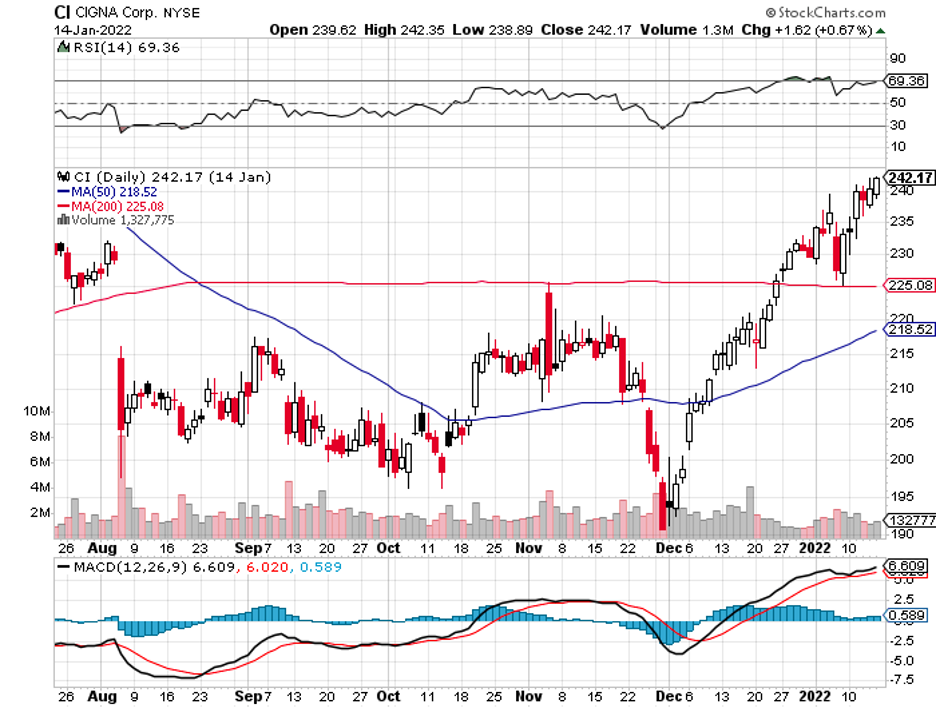
Here’s where Cigna (CI) comes in.
Cigna is one of the handful of companies that are looking into integrating blockchain into their business, such as accepting cryptocurrencies as an additional form of payment.
Needless to say, investing in Cigna would offer you exposure to this new and emerging blockchain technology sans the risk that comes with every new technology.
This is a unique move considering that health insurance stocks are not exactly widely known as proponents of cutting-edge technology.
Aside from Cigna, other providers have been looking into leveraging blockchain to improve their operations. Some names associated with this project include Anthem (ANTM), CVS (CVS), and Cleveland Clinic.
Although blockchain remains in the early innings in terms of its existence in the healthcare industry, investors seeking some exposure would benefit from this reasonably safe option.
After all, Cigna is nothing but a safe stock.
Everyone has practically heard of the company.
Cigna provides Medicare and Medicaid products and insurance coverages not only within the United States but also in some international markets.
Known as a “global health service company,” it has approximately $64.5 billion in marketing capitalization and is considered the fifth-biggest healthcare organization in terms of revenue.
In 2021, it reported over $160 billion in revenue and has managed to rake in profits consistently.
With a net margin of roughly less than 6% of its sales, Cigna has been an investor darling by being consecutively in the black in the last 5 years.
For its 2022 plans, Cigna aims to grow its addressable market to add 3 new states and 93 new countries to reach 1.5 million new customers eventually.
The company also recently secured a new 7-year deal with the US Department of Defense, which would hand over the handling of the healthcare services of roughly 9.6 million active-duty service members to Cigna.
Moreover, Cigna has been working on targeting high-margin sectors like specialty pharmacies.
One of these businesses is Accredo, which manages individuals suffering from complex and hard-to-treat chronic ailments. These conditions include HIV, hepatitis C, and even cancer.
These types of illnesses demand a lifetime’s worth of medications with astronomical price tags. Clearly, being able to get a foothold in this segment would open up a lucrative revenue stream for Cigna.
Basically, Cigna is not your typical flashy stock that gains much attention from the market or the news. Nonetheless, it’s a solid pick that never fails to get the job done.
If anything, investing in Cigna would mean buying and forgetting about it while you collect a stable dividend yield of roughly 2% from this healthcare provider—a solid yield that’s better compared to the 1.3% average of the S&P 500.
So, for cryptocurrency fans, buying Cigna shares would simply be a way to diversify into a sector where you won’t really anticipate that much bullishness on blockchain.