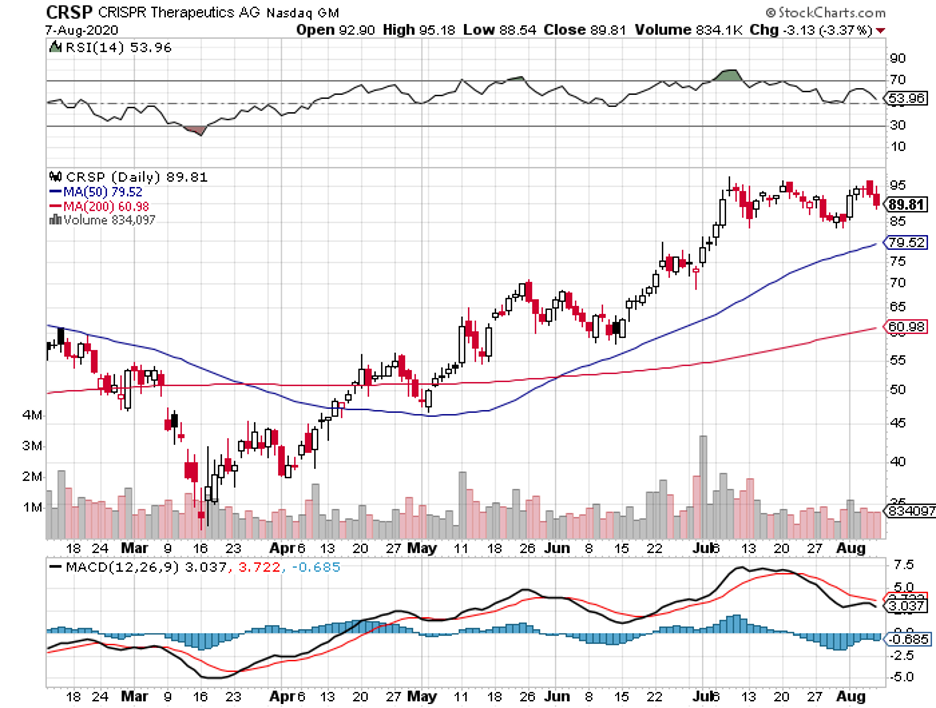
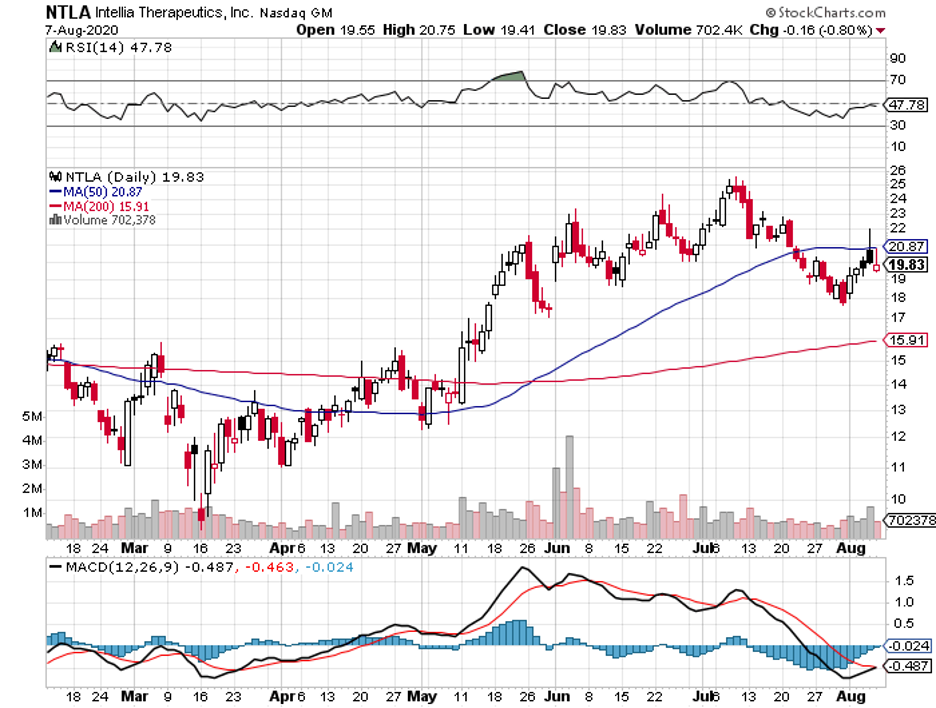
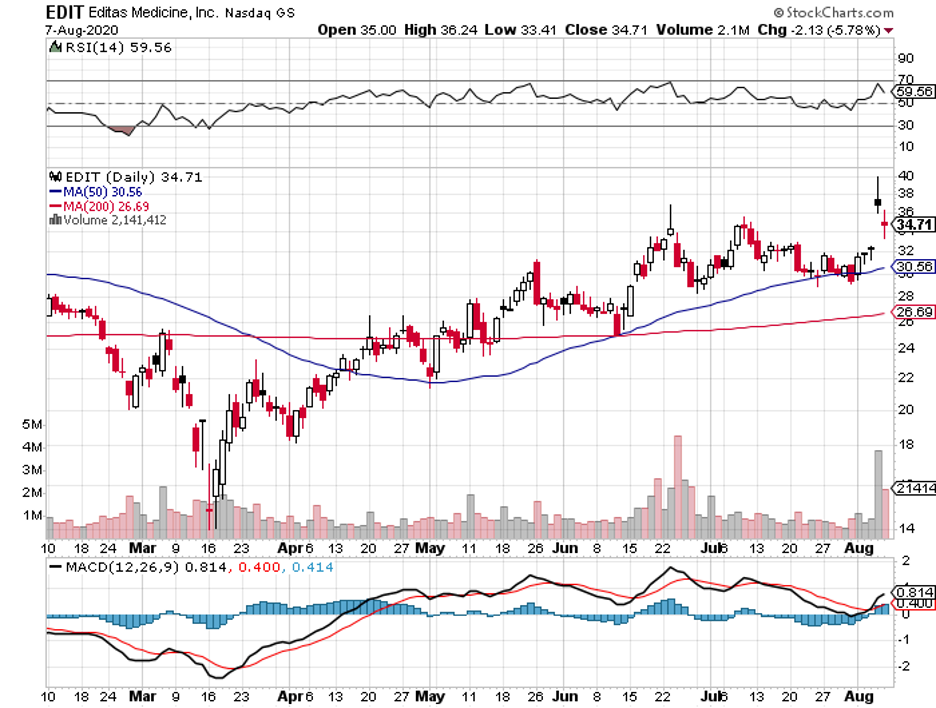
If you still remember the news about the flash recovery from COVID-19 of then-President Trump during the campaign period last year, then you know that the express cure was not delivered by any of the vaccine makers that were all the rage at the time like Moderna (MRNA), Novavax (NVAX), BioNTech (BNTX), or even Pfizer (PFE).
Instead, the cure was credited to a lesser-known cocktail of antibodies, called REGEN-COV, developed by Regeneron (REGN).
Recently, the same treatment was used in Germany in response to the shortage of COVID-19 vaccines and the demand for alternatives.
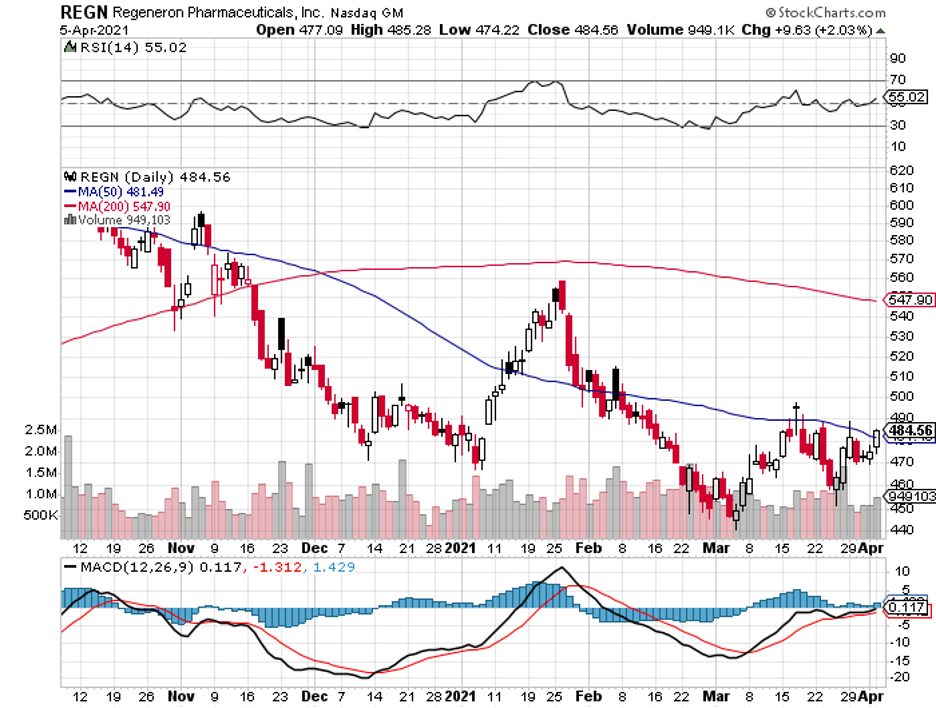
Despite the promising results and the highly publicized effects of Regeneron’s treatment, the company’s share price still hasn’t shown any meaningful upside.
Nonetheless, Regeneron still secured some agreements for REGEN-COV.
Based on the June 2020 agreement of Regeneron with the US government, the company expects to sell $260 million worth of REGEN-COV in the first quarter of 2021 for a fixed number of orders.
For the second quarter of 2021, though, the two parties set different terms for their deal.
Under these new terms, the US government will pay per dose regardless of REGEN-COV’s dose size.
Given the latest numbers from Regeneron’s trials, this could mean lower costs for the company.
Data from the clinical trials showed that REGEN-COV had the same effectiveness at the lower 1,200 mg dosage compared to the currently approved amount by the US FDA, which is 2,400 mg.
In fact, Regeneron’s treatment is reported to be as effective as the COVID-19 antibody therapies developed by Vir Biotechnology (VIR) and even Eli Lilly’s (LLY) candidate.
Looking at the positive results from Regeneron’s Phase 3 trials for REGEN-COV, it’s reasonable to expect higher sales than previously estimated.
Now, Regeneron shared that it aims to supply 1.25 million doses of the COVID-19 antibody therapy at the lower but equally effective 1,200 mg dose level.
If the FDA agrees to this emergency use authorization request, then Regeneron will be able to supply twice the number of COVID-19 doses.
If it delivers these doses by June 30, the US government will buy them for $2.6 billion regardless of the dosage used.
On average, Regeneron is expected to generate roughly $2.9 billion in sales for its COVID-19 antibody treatment.
Meanwhile, if REGEN-COV gains full FDA approval and gets marketed commercially, then the treatment can rake in at least $3.5 billion and peak at $5 billion this year alone.
Outside its COVID-19 program, Regeneron actually recorded better-than-expected results last year despite the pandemic ravishing the economy.
For example, there was a rebound in demand for its top-selling Eylea, with sales of the wet age-related macular degeneration (AMD) drug rising by 10% in the fourth quarter of 2020 to reach a total of $1.34 billion.
Bolstering the dominance of Eylea in the AMD market and to combat emerging competitors like Roche (RHHBY) with Faricimab and Novartis (NVS) with Beovu, Regeneron is looking to expand the drug’s application to cover more age groups.
Meanwhile, another bestseller, Dupixent, reached $1.17 billion in sales last year.
This is an impressive climb for the atopic dermatitis medication, which was developed with Sanofi (SNY), since it only recorded $751.5 million in the same period in 2019.
That indicates roughly 75% growth, with over a million prescriptions written for Dupixent in the US alone.
However, only 6% of those eligible patients have been treated with Regeneron’s product thus far.
This means that Dupixent has a lot of room to grow, with this drug estimated to reach peak sales at $12.5 billion.
Needless to say, Dupixent is quickly transforming into a blockbuster treatment.
Since its approval for eczema in 2017, this drug has expanded its indication to cover moderate-to-severe atopic dermatitis not only among teens but also children. Notably, Dupixent holds a monopoly for this application to children.
Another revenue stream for Regeneron is its oncology sector led by Libtayo.
In 2020, net sales of this skin cancer treatment reached $348 million, showing an impressive 80% growth.
To date, Regeneron has at least 12 oncology treatments under clinical development.
In terms of the bottom line, Regeneron exceeded the expectations of $8.38 and reported adjusted earnings per share of $9.53 instead.
As vaccine rollouts continue to be a priority, it’s safe to say that the worst of the COVID-19 is just about in sight.
Consequently, investors are now looking into recovery and stocks that appear to be good buys when the coronavirus eventually becomes a thing of the past.
Regeneron is one of the attractive buys so far. While it has been underperforming in the past weeks, its business actually looks to be in great shape even if the pandemic goes on for longer.