Another biotechnology company is cashing in on its COVID-19 vaccine efforts: CureVac (CVAC).
CureVac, which has a market capitalization of $9.9 billion, is hoping to follow the footsteps of Moderna (MRNA) and BioNTech (BNTX).
Earlier this year, both small-cap companies saw their value skyrocket, with Moderna now reporting a market capitalization of $27.3 billion while BioNTech is at $16.3 billion.
While the jump in their market capitalization is definitely newsworthy, what is even more impressive is that neither company has a product out in the market today. That is, up until the pandemic struck.
Now, CureVac is looking into raking in the same benefits from its own COVID-19 vaccine work.
Here is a snapshot of how well this stock is doing so far.
CureVac, which raised $213.13 million in its IPO, initially priced its shares at $16 each, started trading at $44 per share and ended the day at $55.90 per share.
The week after, CureVac shares started trading at $79.33 in the premarket hours of Monday, with the price expected to reach an all-time high of approximately $85 per share.
Aside from the Bill and Melinda Gates Foundation, CureVac also attracted backing for its COVID-19 vaccine candidates from the German government and GlaxoSmithKline (GSK). So far, the company has recorded $640 million in funding for its coronavirus program.
What we know about CureVac’s vaccine candidate is that it utilizes the same mRNA-based technology as Moderna and Pfizer (PFE).
While the newly minted biotechnology company is behind competitors, the results of their study are expected to be released by the next quarter.
Prior to prioritizing its COVID-19 vaccine work, CureVac has been focusing on developing cancer and rare disease treatments.
CureVac is also developing CV8102, which is a treatment that can target four different kinds of tumors.
Another frontrunner in its pipeline is CV7202, which is its rabies drug candidate. Its second-generation lipid nanoparticle (LNP) flu vaccine, called CV6301, is also a promising treatment.
Apart from CureVac, another small-cap biotechnology company has been competing against the COVID-19 vaccine frontrunners like AstraZeneca (AZN) and Johnson & Johnson (JNJ).
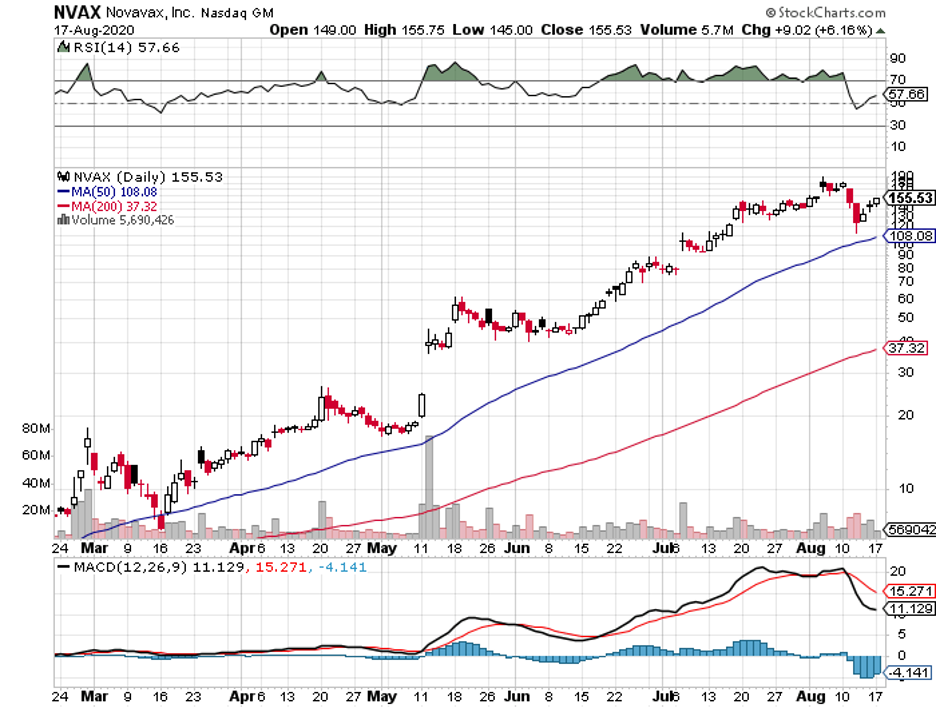
Earlier this month, Novavax (NVAX) announced the launch of the Phase 2B clinical trial of its COVID-19 vaccine.
The trial for the coronavirus vaccine, called NVX-CoV2373, is set in South Africa and is anticipated to not only provide the company with a larger group but also test the vaccine’s efficacy in an environment where the disease is currently surging.
Although Novavax is also behind the leaders, the level of transmission rate in South Africa, which accounts for half of the COVID-19 cases in Africa, is expected to provide the company a better chance of evaluating its candidate.
Other than that, Novavax has also secured manufacturing deals that can handle more than 2 billion doses.
Novavax has been working on a COVID-19 vaccine since February, with the company receiving $388 million in funding from the Coalition for Epidemic Preparedness Innovations.
By July, the company received a $1.6 billion investment from the US government courtesy of Trump’s Operation Warp Speed project.
If Novavax’s vaccine candidate earns approval, then the company could realistically expect over $10 billion in annual sales.
Riding the momentum, Novavax has also been working on a flu vaccine candidate, called NanoFlu, which can record as much as $1.7 billion in yearly sales.
With the current financial climate, the unprecedented demand for a vaccine will unsurprisingly drive the shares of companies like Novavax and CureVac even higher.
However, it is better to err on the side of caution when it comes to these ultra risky biotechnology companies.
The biotechnology industry has no shortage of investors on the lookout for stocks that can easily make them filthy rich. Although these high-profile stocks can definitely result in massive gains, there are still a number of critical caveats to bear in mind.
While waiting for the actual candidates to get launched, it is safer to bet on tested and proven businesses for now and perhaps dip your toe in the unfamiliar water currently dominated by these small-cap biotechnology companies.