The biotechnology and healthcare sectors have become attractive investment targets for investors who recognize the value and essence of these industries along with the possible risks associated with them.
While not all companies in these areas are great investments, some offer remarkable growth opportunities.
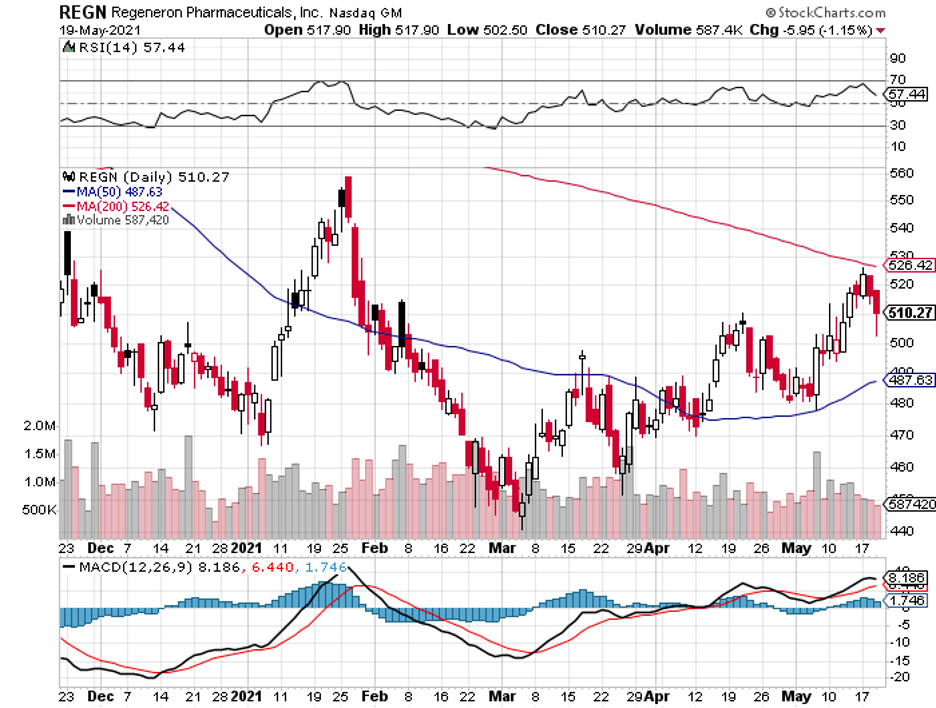
One company worth considering is Regeneron (REGN), with its strong and stable investment thesis and steady organic growth.
Regeneron joins the ranks of Pfizer (PFE) and Johnson & Johnson (JNJ) as one of the handful of biopharmaceutical companies to release solid first quarter results this 2021 compared to other big names in the industry, including Amgen (AMGN), Bristol Myers Squibb (BMY), Gilead Sciences (GILD), Merck (MRK), and Eli Lilly (LLY).
The New York-based company reported a 38% boost in its revenue compared to the same period in 2020, reaching $2.5 billion for the first quarter of 2021 alone.
Virtually all of Regeneron’s products generated solid growth during this period, with the company’s COVID-19 antibody cocktail REGEN-COV delivering the highest sales at $262 million.
To underscore just how significant REGEN-COV is to Regeneron this quarter, its absence from the roster would take away 18% from the company’s overall revenue growth.
Riding the momentum of its COVID-19 program, Regeneron has developed Inmazeb, which is a treatment for Ebola virus infection.
Aside from its COVID-19 antibody cocktail, Regeneron also saw an impressive boost in the performance of its atopic dermatitis drug Dupixent.
Dupixent, which Regeneron sells in partnership with Sanofi (SNY), generated $1.26 billion in sales in the first quarter, showing off a notable 48% increase from its 2020 report.
Although Dupixent is a shared product with Sanofi, this dermatitis drug holds incredible promise for Regeneron.
To date, only 6% of eligible patients are being treated with Dupixent. This indicates a massive space that is yet to be explored by both companies.
Taking into consideration the pace at which Dupixent has been growing so far, this drug is projected to peak at roughly $12.5 billion in sales in the coming years.
Another high-selling drug for Regeneron is wet age-related macular degeneration (AMD) treatment Eylea.
Sales for this drug, which was developed in collaboration with Bayer (BAYRY), went up from $1.2 billion in the first quarter of 2020 to $1.3 billion this year.
The increase in sales for Eylea is a welcome surprise for both Regeneron and Bayer, especially since more and more competitors are attempting to topple the drug as the top product in the niche.
Cornering the AMD segment is an attractive venture for any biopharmaceutical company.
After all, Eylea generated $4.9 billion in sales in 2020 from the US market alone.
Thus far, two main competitors have come forward as the strongest.
One is Novartis (NVS), which released Beovu in 2019.
The second, and possibly the stronger competitor between the two, is Roche (RHHBY) with Faricimab.
To ensure its dominance in the AMD market, Regeneron has been expanding the use of Eylea.
The latest development is the drug’s enrollment in the Phase 3 program, which would allow extended periods in between treatments but still deliver the same level of efficacy and safety.
Aside from these, Regeneron is looking into additional revenue streams ahead.
One growth segment is its oncology program, particularly its cancer drug Libtayo, which may soon be marketed to cover a fourth type of cancer.
Regeneron aims to submit Libtayo for review as a treatment for advanced cervical cancer.
On top of this, the drug is also a strong contender in the development of several antibody treatments.
Thus far, the company has 12 oncology antibodies under clinical development.
Overall, Regeneron’s strong results for the first quarter of 2021 highlighted its continuous evolution into a company carrying multiple and diverse portfolios of products and pipeline programs that address an extensive range of serious diseases, from COVID-19 and rare diseases to cancer.