Biotechnology stocks have been going through a rough patch in the past weeks.
These previously unstoppable stocks have trailed the same path as the market in a sell-off, which has some of their investors fighting to keep calm.
In fact, the iShares Biotech Nasdaq ETF slid over 9% in the past month—a fall that affected some players who experienced all-time highs or enjoyed four-digit gains in 2020.
Inasmuch as all these sound discouraging, the decline might actually offer an opportunity.
Now, investors can snatch up some excellent stocks at pre-pandemic prices.
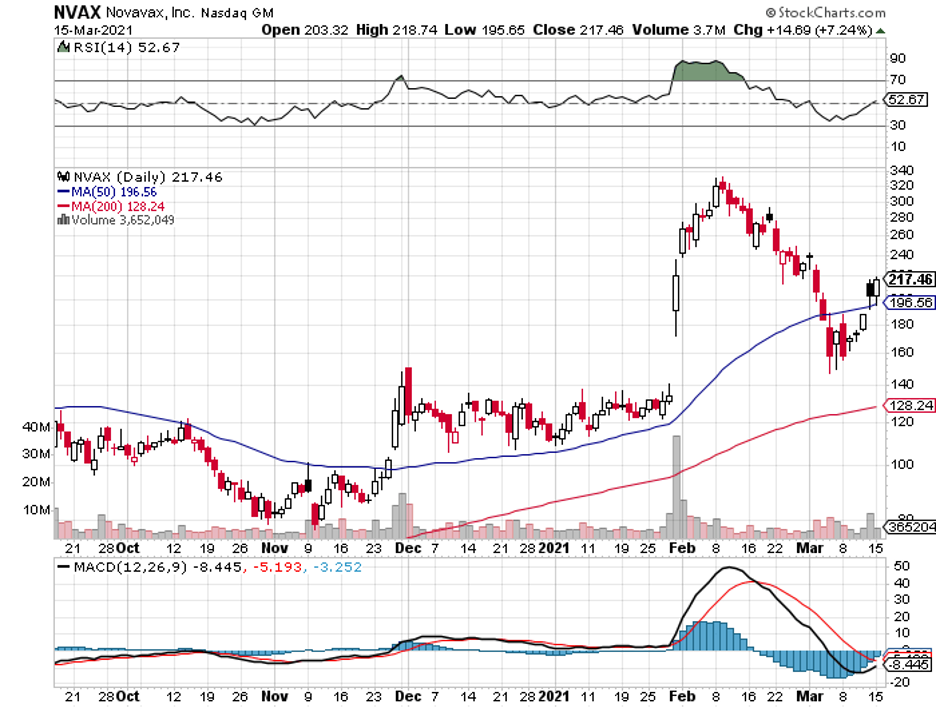
Among the biotechnology stocks that have dropped more than 28% to date from their latest high points, I find Novavax (NVAX) to be one of the most promising.
Novavax rose to over 2,700% in 2020, with the stock extending its gains well into 2021.
In the first five weeks of the year, Novavax jumped 186% following positive data from the Phase 3 trial of its COVID-19 vaccine, NVX-CoV2373, in the UK.
NVX-CoV2373 is widely anticipated to be the next COVID-19 vaccine candidate to win major regulatory approval.
Its Phase 3 study in the UK showed that NVX-CoV2373 was 96.4% effective against mild, moderate, and even severe cases of COVID-19 caused by the original strain of the coronavirus.
When tested against the “UK variant,” NVX-CoV2373 showed 86.3% efficacy.
Meanwhile, NVX-CoV2373 was found to be 55.4% efficacious against the “South African” variant.
Overall, NVX-CoV2373 proved to be 100% effective in protecting patients from hospitalization and death. More importantly, the vaccine didn’t cause severe side effects.
Despite the promising results released, the biotech stock has slipped 46% since early February.
While some investors fret over the fact that rivals like Pfizer (PFE), Moderna (MRNA), and Johnson & Johnson (JNJ) have already started production and shipment of their vaccines, the developers of NVX-CoV2373 say there’s nothing to worry about.
NVX-CoV2373 doses are readily available and can be shipped as soon as Novavax gains regulatory approval.
More reassuringly, this vaccine candidate can be stored for months without any special handling.
While it hasn’t landed as many orders in the United States as its counterparts, Novavax has been securing its spot in the international markets.
The company has landed orders for roughly 200 million doses of NVX-CoV2373 for Canada, Australia, Switzerland, New Zealand, and the UK. It also has an agreement to supply 1.1 billion doses to the Serum Institute of India.
On top of these, Novavax has signed a deal to supply over 1 billion doses to COVAX, which is a global project initiated to secure fair access to vaccines for all countries.
Novavax has been boosting its manufacturing capacity as well, with the company ramping to produce over 2 billion doses of NVX-CoV2373 every year by mid-2021.
By comparison, Moderna is estimated to produce about 700 million to 1 billion doses this year.
In terms of revenue, Novavax hasn’t definitely discussed its pricing. What we know so far is the price paid by the US, which is $16 per dose.
Back of the napkin math says that brings the total to $4.8 billion for the orders from the US and the other countries so far this year and excluding the COVAX deal since the pricing might be substantially lower.
This is a massive revenue for a biotech company that doesn’t even have a product revenue yet.
Another exciting prospect is Novavax’s pipeline.
Right now, the company has another vaccine that can become a great revenue source: NanoFlu.
Before the pandemic broke, NanoFlu was actually the major reason investors flocked towards Novavax.
With its overwhelming performance against Sanofi’s (SNY) own FluZone Quadrivalent, NanoFlu has been slated for a myriad of commercial possibilities.
These include developing it as a combination vaccine with NVX-CoV2373 as well as with Novavax’s other vaccine candidate, with its experimental respiratory syncytial virus (RSV) vaccine.
Another program is looking into combining all three vaccines together.
Riding the momentum of its success with NVX-CoV2373, Novavax is also planning to develop vaccines for other coronavirus variants.
This could include a bivalent vaccine program, which is expected to commence by June 2021.
All these programs are positioning Novavax as a dominant leader in the vaccine market.
If you haven’t considered Novavax before, then now is a good time to look into the stock. This is a company that has billions in locked-in revenues coming in this year alone.
Basically, buying Novavax stock would get you revenue in the near future, new products that will generate additional sales, and pipelines that offer growth—and if you buy the stock on the dip, you’re getting all these at a bargain.