No matter how you look at it, the stock market is definitely facing serious volatility these days. How long this uncertainty will last and whether it’s a sign of a looming market crash or correction is anybody’s guess.
On a positive note, the current situation will not cause panic to long-term investors. After all, it’s not right to base stock-buying decisions on the market’s behavior over the course of a few days, weeks, or even months.
Meanwhile, if a major bear market is on the horizon, then this could present a good opportunity to add resilient and recession-proof stocks to your portfolio.
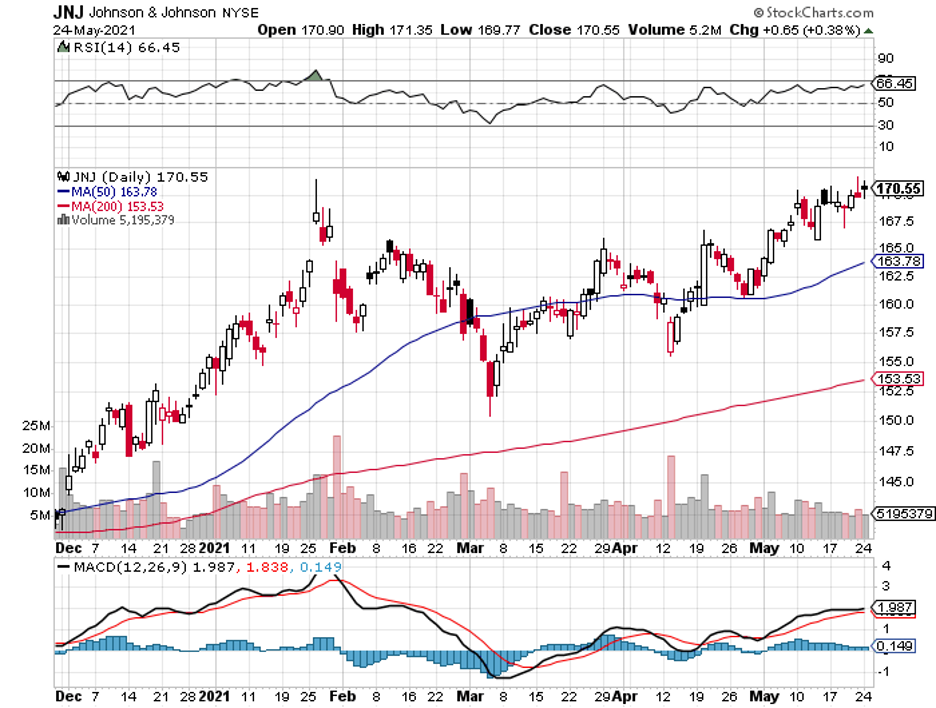
In the biotechnology and healthcare sector, one stock comfortably fits the mold, and that's Johnson & Johnson, JNJ.
In terms of market capitalization, JNJ is one of the biggest—if not the biggest—pharma companies in the world, weighing in at roughly $450 billion.
It also holds an undisputed status as a Dividend King, which is a title granted to those companies that increase their payouts annually and consistently over the course of 50 years.
Actually, JNJ’s dividend-hiking streak has stretched to 59 straight years—and it doesn’t seem to be ending anytime soon.
To date, its quarterly dividend per share jumped by 5% from $1.01 to reach $1.06.
That’s why it comes as no surprise that it’s one of the stocks that investors come running to for safety and stability during periods of volatility.
In its first quarter earnings report in 2021, JNJ showed off by outperforming revenue expectations of $21.98 billion to record $22.32 billion instead.
Its EPS also beat estimates of $2.34 and instead reported $2.59. Despite the less-than-stellar condition of the US economy in the past months, JNJ still managed to boost its sales in the first quarter and increased its sales by 7.9% year-over-year.
All of JNJ’s core business segments also expanded their revenues this quarter.
For instance, its Janssen pharmaceutical arm, which was in charge of its COVID-19 vaccine, saw a 9.6% year over year increase in sales to reach $12.19 billion.
Even its medical devices segment experienced an improved performance with a 7.9% bump to record $6.57 billion for this quarter alone.
Looking at the programs in its pharmaceutical division, it’s clear that JNJ has a strong focus on six areas: cardiovascular, pulmonary hypertension, immunology, neuroscience, metabolism, and, of course, oncology.
In fact, three of JNJ’s pharmaceutical treatments raked in more than $4 billion in sales in 2020.
The list was topped by Stelara, which is a drug for Crohn’s disease, psoriasis, ulcerative colitis, and psoriatic arthritis, at $7.7 billion.
It was followed by multiple myeloma treatment Darzalex at $4.2 billion.
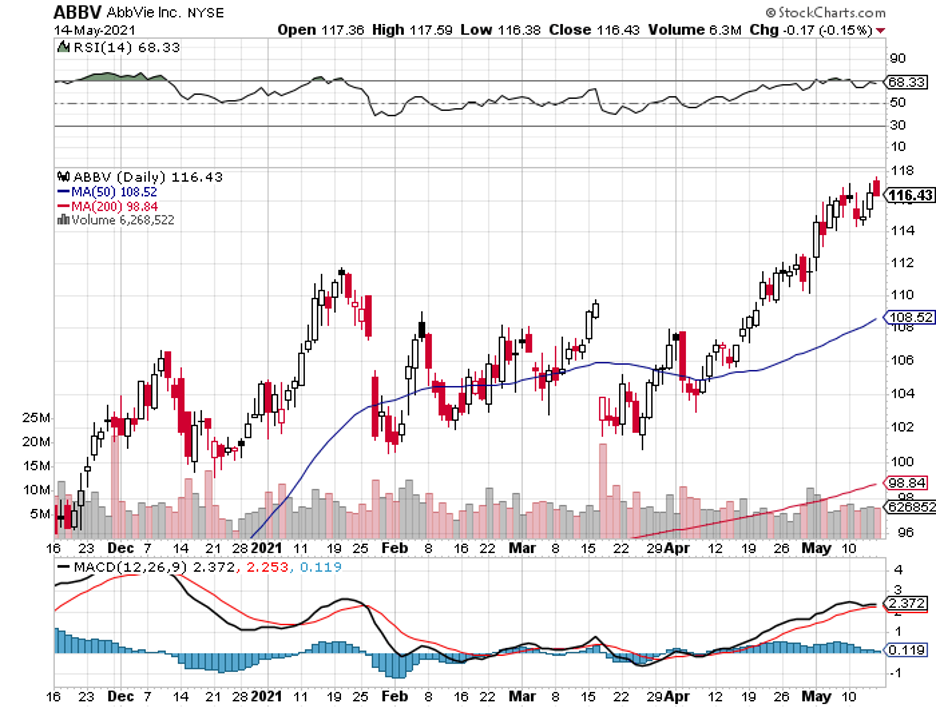
The product of its collaborative work with AbbVie (ABBV), blood cancer drug Imbruvica, rounds up the list at $4.1 billion.
The sheer size and financial power of JNJ offer the company extensive M&A opportunities—and it’s definitely taking advantage of that to continue boosting its revenue streams.
In August 2020, amid the COVID-19 pandemic, JNJ acquired Momenta Pharmaceuticals for $6.5 billion. This all-cash transaction added a slew of drug candidates that enhanced JNJ’s immune-mediated and rare disease pipeline programs.
Jumping into the telehealth bandwagon, JNJ has invested in Madison Thirty around the same time last year as well.
Much like Teladoc (TDOC), this small telehealth company has also attracted attention since it started and thus far raised $70 million in funding.
Boosting its presence in the merging world of technology and medicine, JNJ recently revealed its six-armed robotic surgical assistant, Ottava.
Basically, Ottava will be a high-tech guidance system and assistant to surgeons in operating rooms.
Throughout its history, JnJ has proven itself to be a top biopharmaceutical stock —full stop.
A global leader in the healthcare industry, JNJ is one of only two corporations that hold an AAA credit rating from Standard & Poor. The other company is Microsoft (MSFT).
It has been generating record profits and boosting its dividends. More importantly, investors can expect JNJ stock to serve as a healthy long-term wealth generator.
It prides itself on a strong triad of business segments that continuously drive growth: consumer health, pharmaceuticals, and medical devices.