As we gradually reach the pinnacle of biotechnology formation, a war is brewing in the life sciences world.
This can be one of the most exciting times for medical innovations for patients. Meanwhile, investors can be picky when picking where to put their money.
Even up-and-coming scientists can seize the opportunities to lay the groundwork for their own dream organizations.
At the same time, those aspiring to climb the corporate ladder have better chances at becoming CEO without the need to slog through the biopharma sector and scramble for whatever opening is available.
However, as more and more companies launch practically every day, claiming to offer groundbreaking and revolutionary breakthroughs, it’s critical to keep in mind that not all biotechs will succeed.
Actually, the number of biotech companies has been steadily rising since 2015.
In that year, 177 firms were formed, with biotech birth rates breaching the 200-per-annum mark by 2017 and 2018.
Seeing as many more have emerged even during the pandemic, it looks like the biotech world won’t be slowing down anytime soon.
Even funding hasn’t been deterred by economic downturns.
From 2015 to 2018, the total funding for biotech companies averaged between $68.6 million to roughly $90.2 million.
After a bustling, record-breaking 2020, the bar leading to 2021 was expectedly high.
Surprisingly, 2021 blew those figures out of the water as private investors opted to raise the bar even higher.
It’s the type of climb that’s truly hard to believe.
Biotechs raised over $22 billion in private funds in 2020 following a sluggish 2019. In 2021, that figure rose to $28.5 billion.
The top earner in these funding rounds last year was China’s Abogen, which took $1 billion in private investors’ money across two rounds.
Abogen is an mRNA-centered firm that’s currently working on a COVID-19 vaccine.
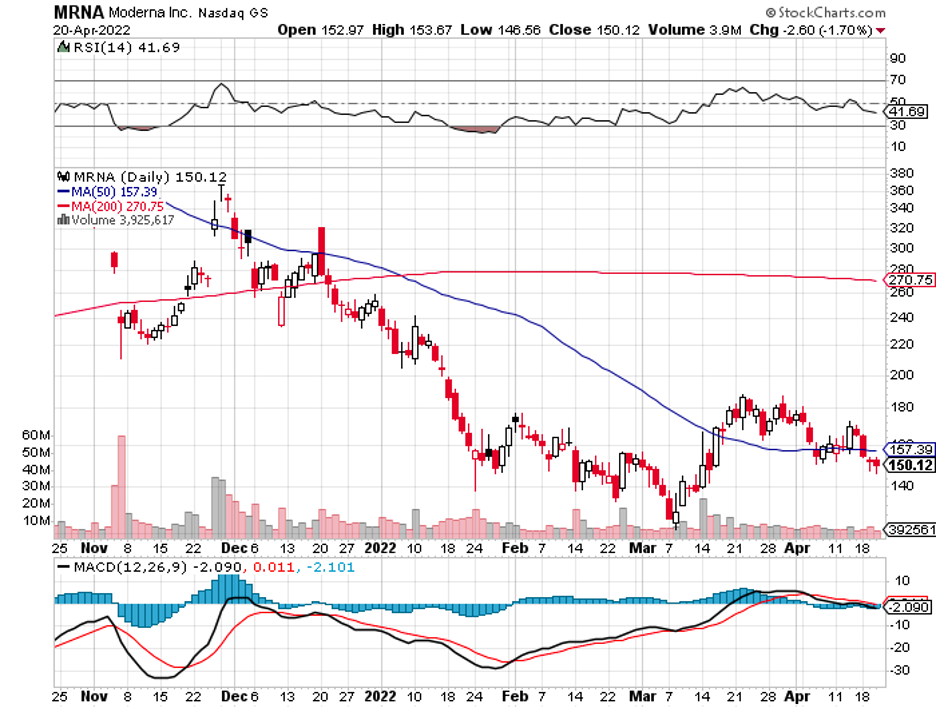
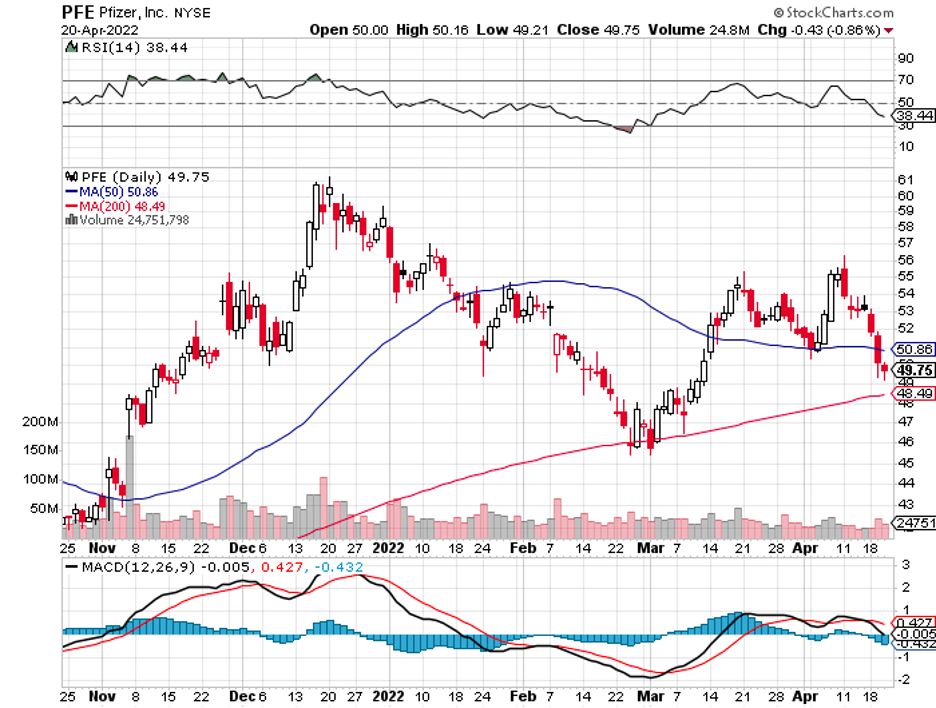
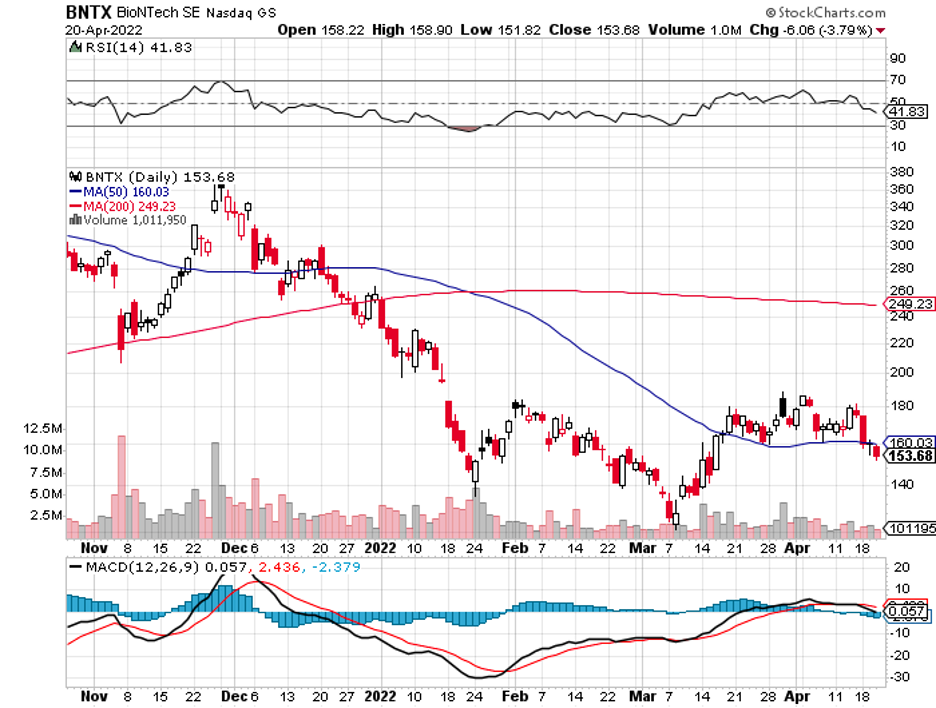
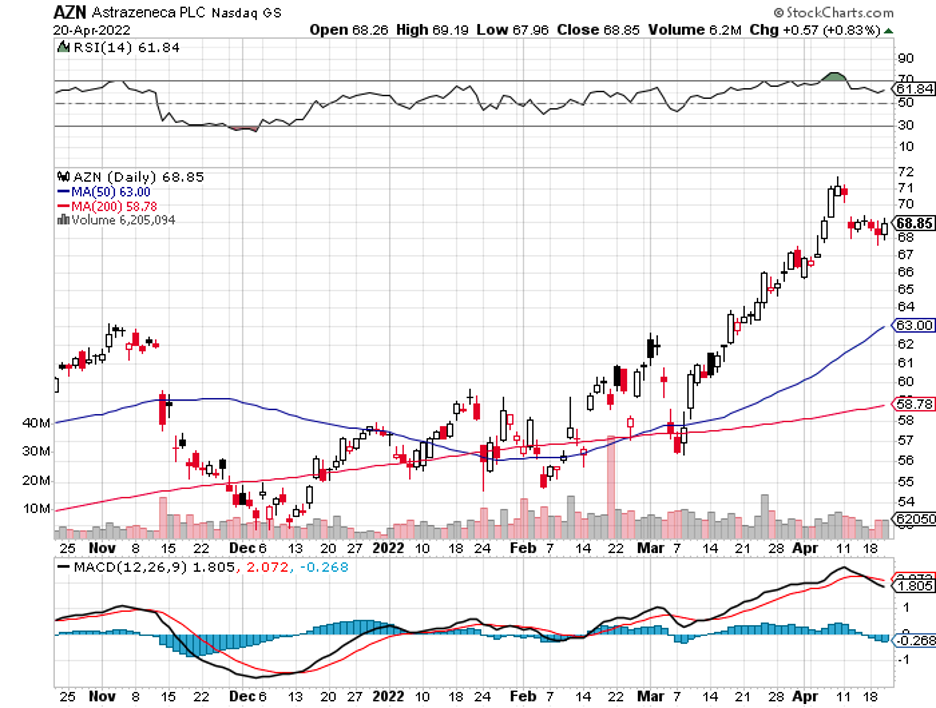
What makes its product different and possibly better than Moderna (MRNA), Pfizer (PFE), BioNTech (BNTX), and AstraZeneca (AZN) is that it would be thermostable. That is, it could be used in areas without access to refrigeration.
Another big winner in 2021 is Massachusetts-based biotech ElevateBio, which aims to be a one-stop shop for cell and gene therapies.
The idea is to develop a technology that fuses its gene-editing platform, cell engineering structure, and manufacturing warehouse into one system to ease and accelerate the drug development process.
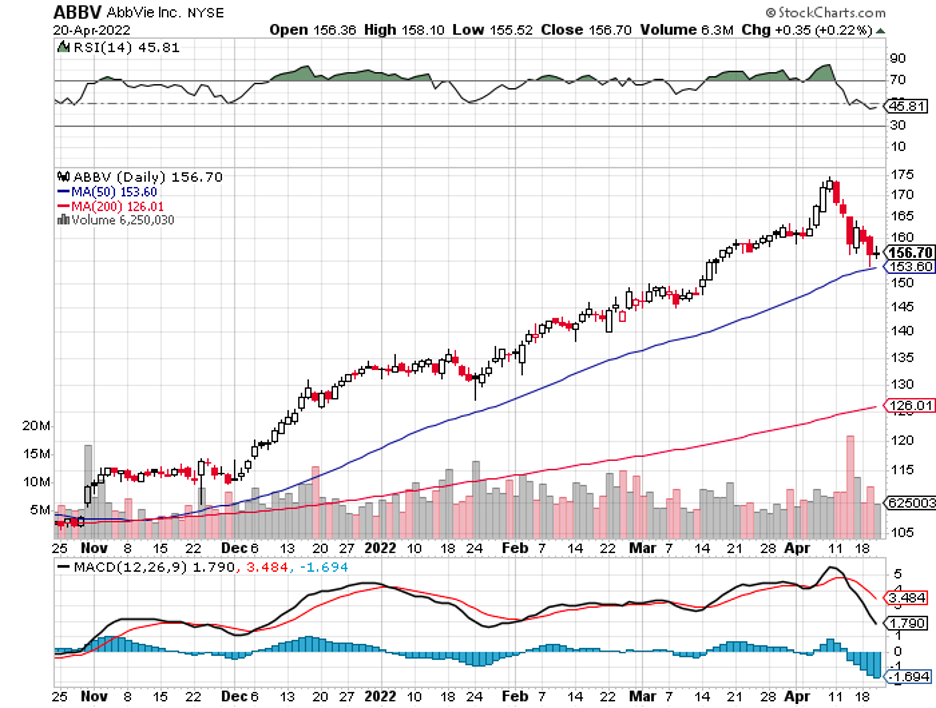
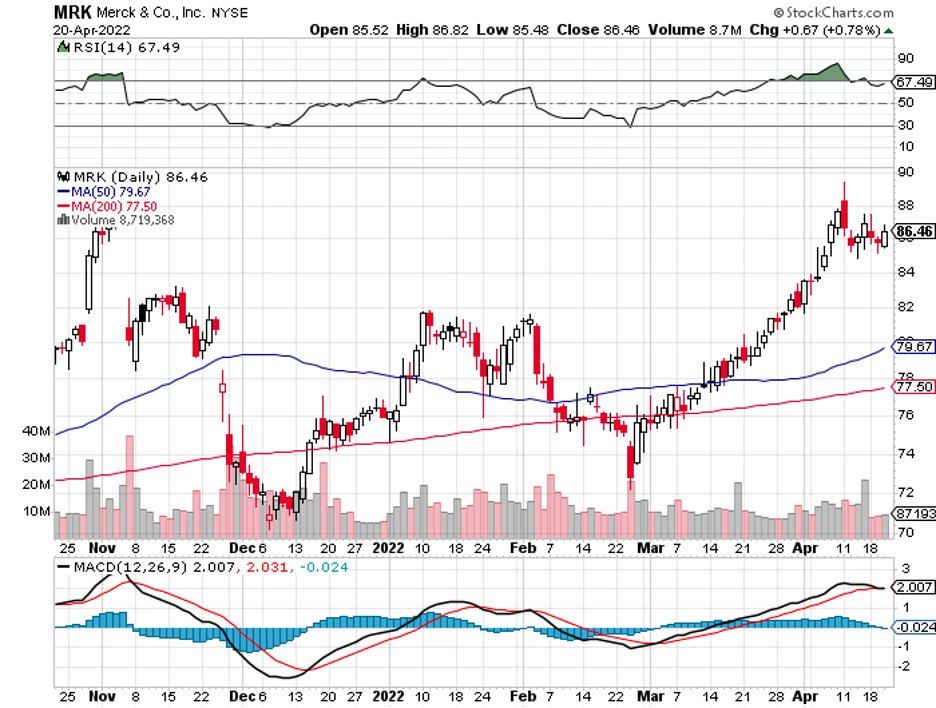
Although not entirely the same, this plan has similarities with the strategies of Big Pharma names like AbbVie (ABBV) and Merck (MRK).
Amid the growing number of biotechs, a key challenge is how to stand out among companies that target the same disease areas. This kind of competition could hamper innovation.
The clearest indicator of success would be receiving approval and being able to launch the products commercially.
Ultimately, the goals are to offer safe and effective treatments and provide value to their shareholders.
Unfortunately, the reality is only a handful of startups do make it all the way to the top.
The more feasible scenario is that bigger businesses would acquire these companies—and that seems to be the case these days.
Alongside the booming biotech formation rate are the increasingly aggressive biotech buyout deals.
We’ve seen this before.
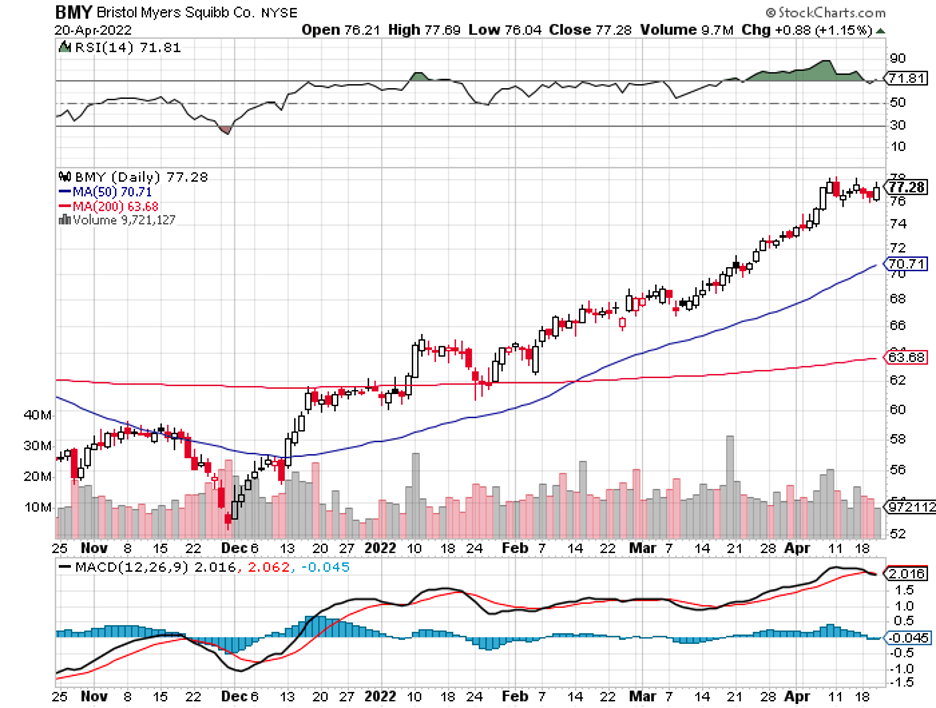
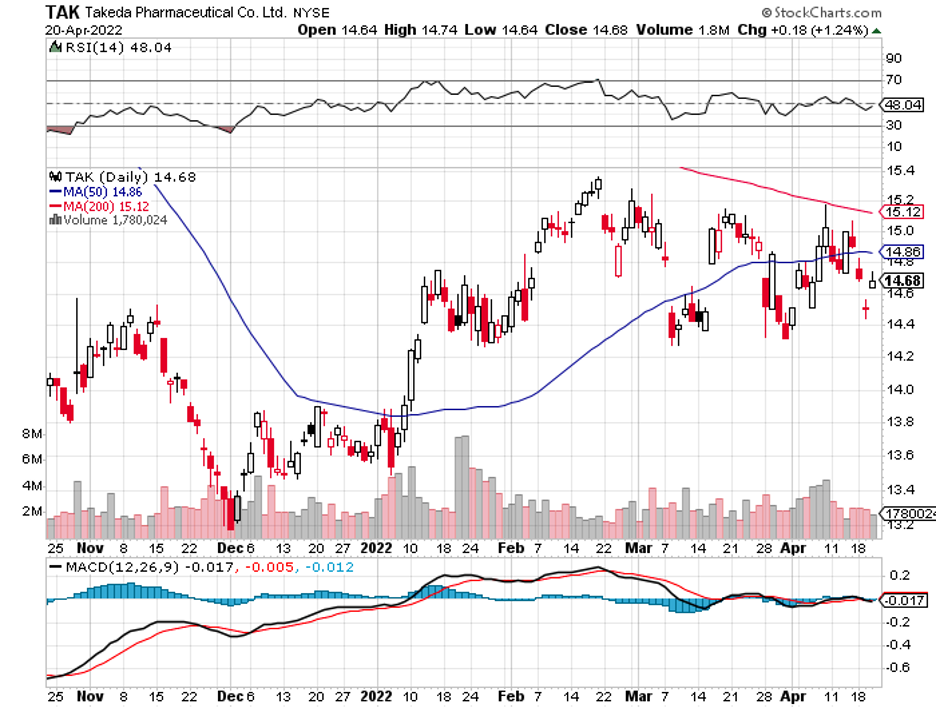
It started in 2019, with Bristol Myers Squibb (BMY) buying Celgene, followed by AbbVie splurging on Allergan and Takeda (TAK) merging with Shire.
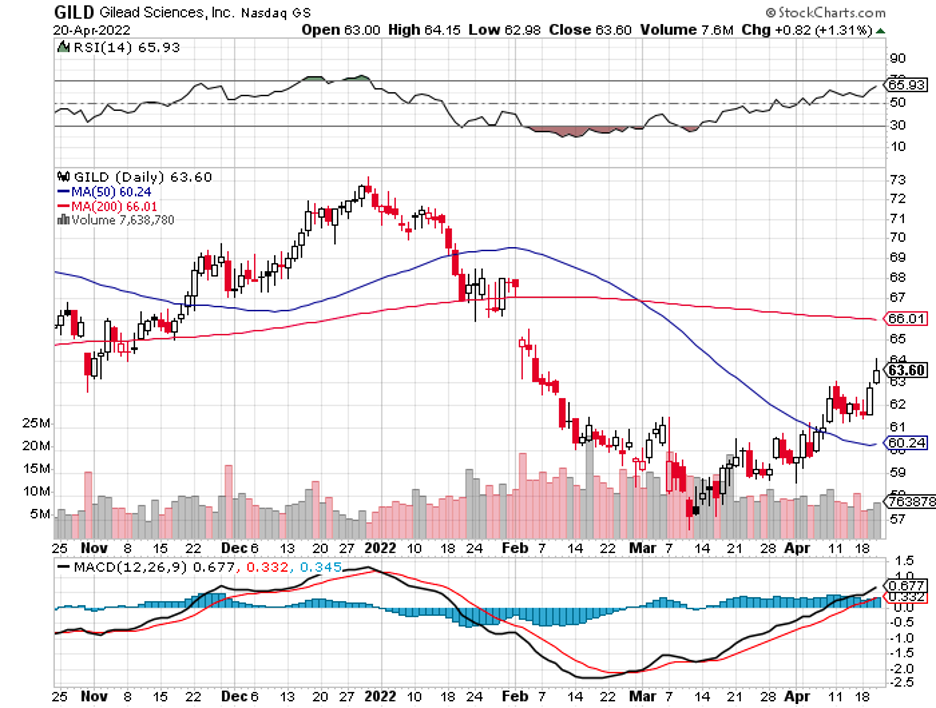
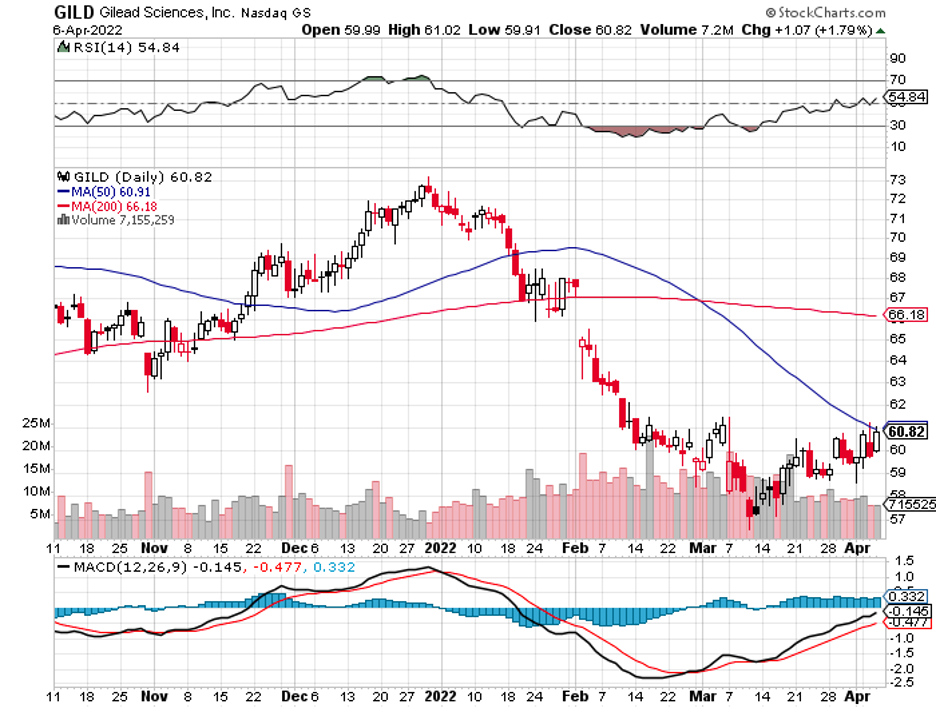
In 2020, AstraZeneca bought biotech superstar Alexion Pharmaceuticals while Gilead Sciences (GILD) snapped up Immunomedics.
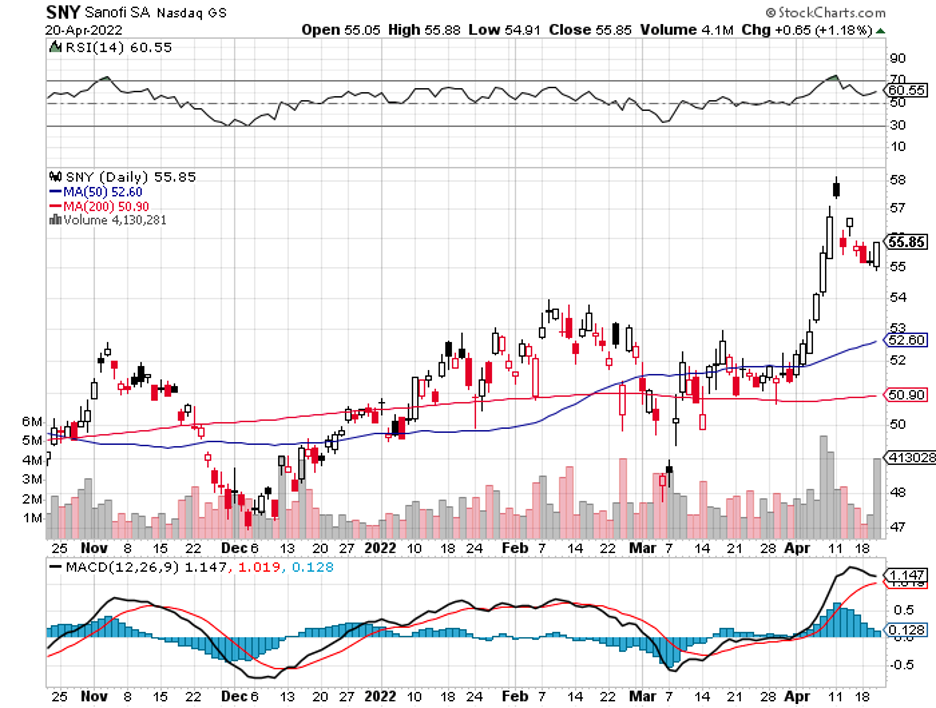
Meanwhile, Sanofi (SNY) stacked its deck with the $3.2 billion acquisition of Translate Bio. As for Merck, this biopharma sneaked in a massive win with an $11.5 billion buyout of Acceleron.
For this year, several names have already been eyed by Big Pharmas.
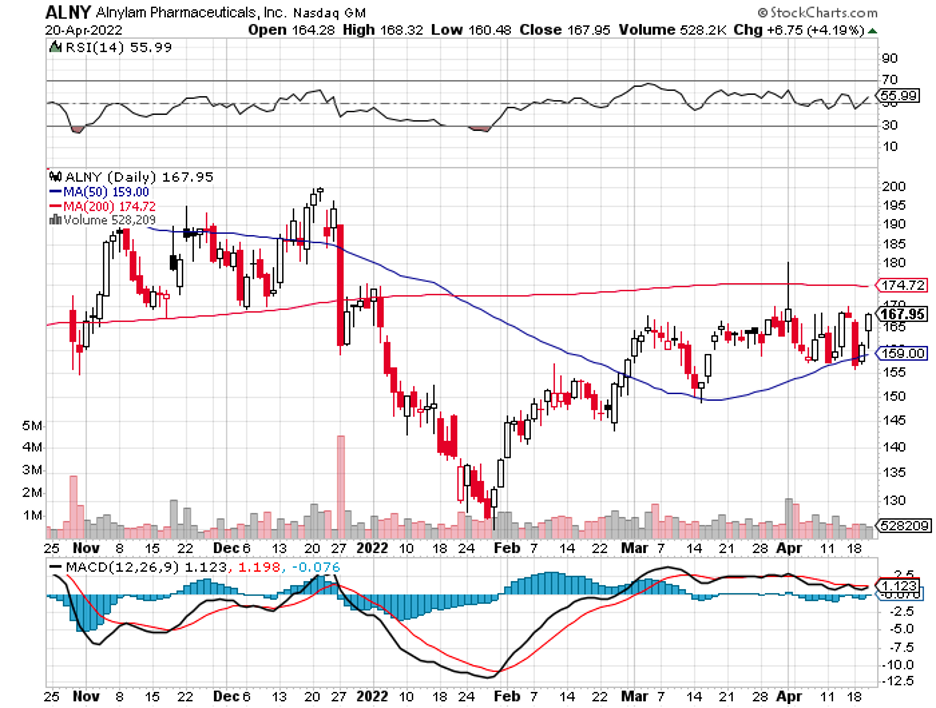
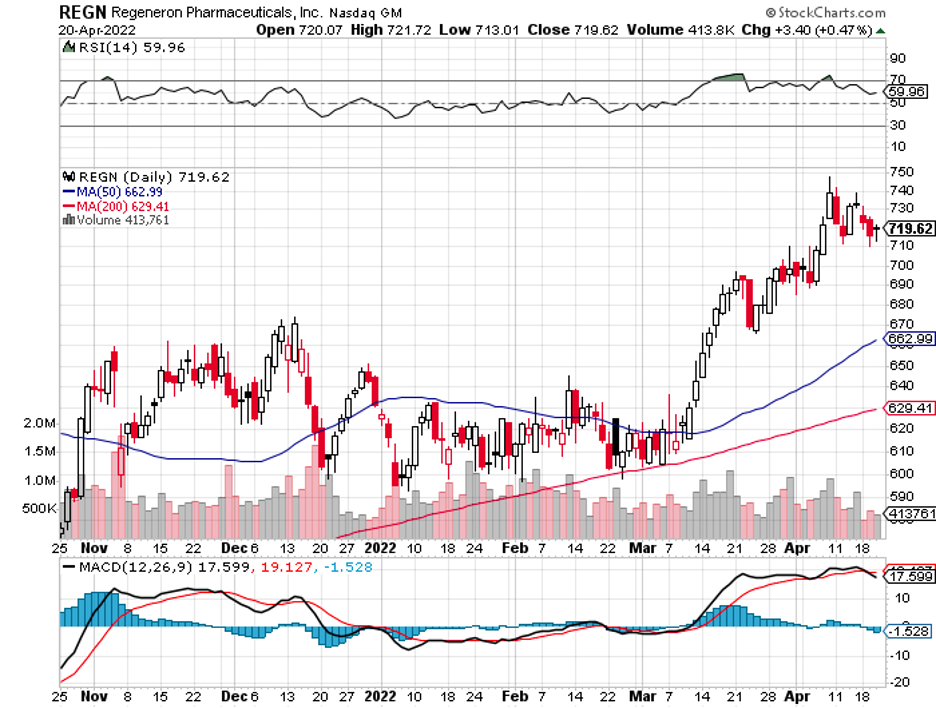
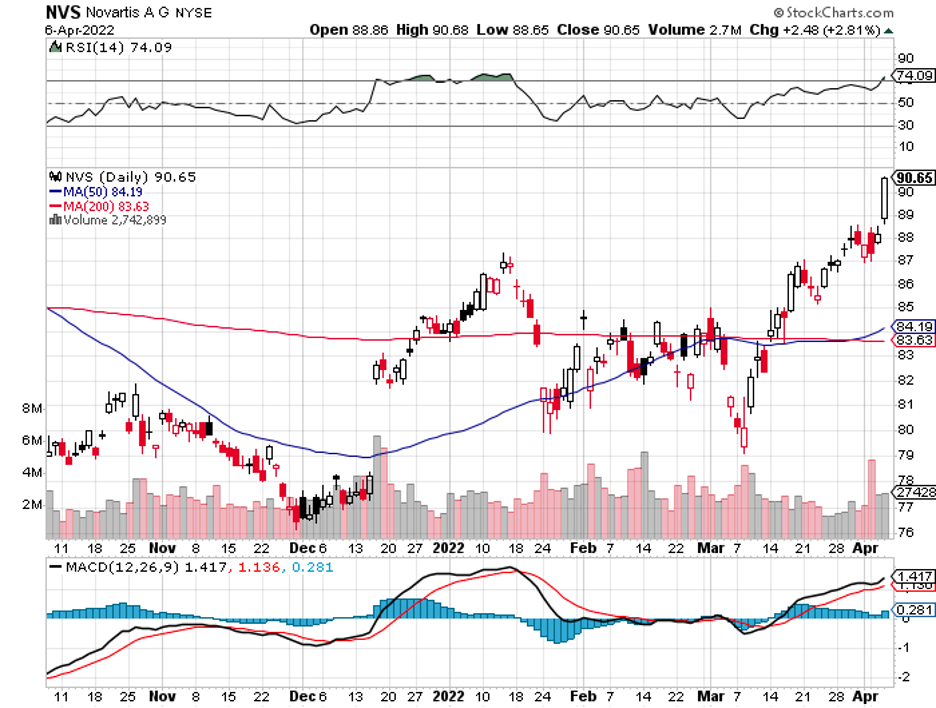
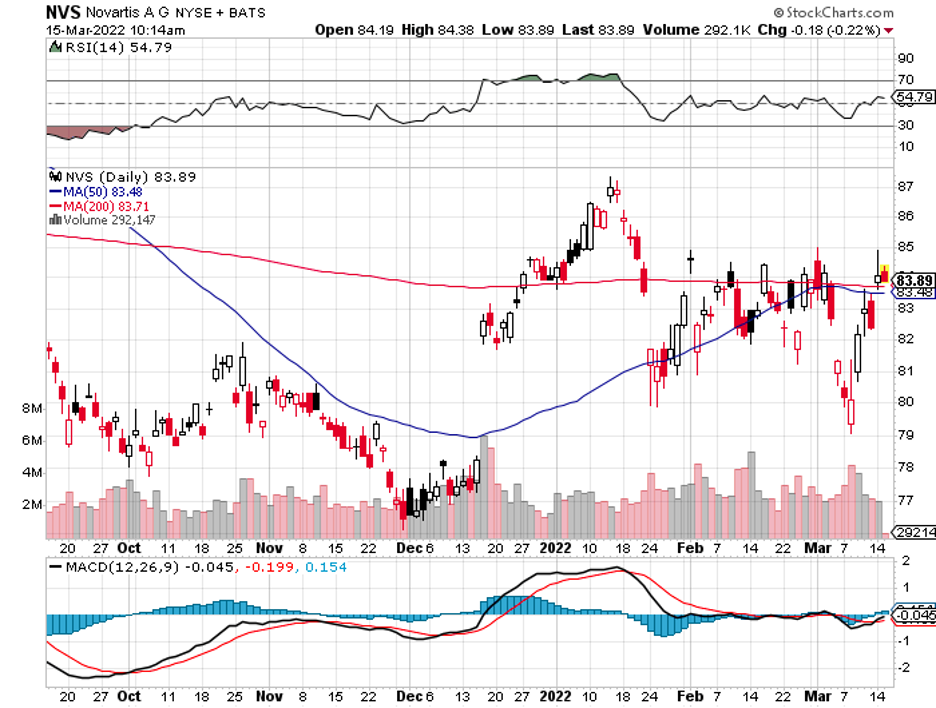
There’s Alynlam Pharmaceuticals (ALNY), an RNA-centered company, which seems to be the target of both Novartis (NVS) and Regeneron (REGN).
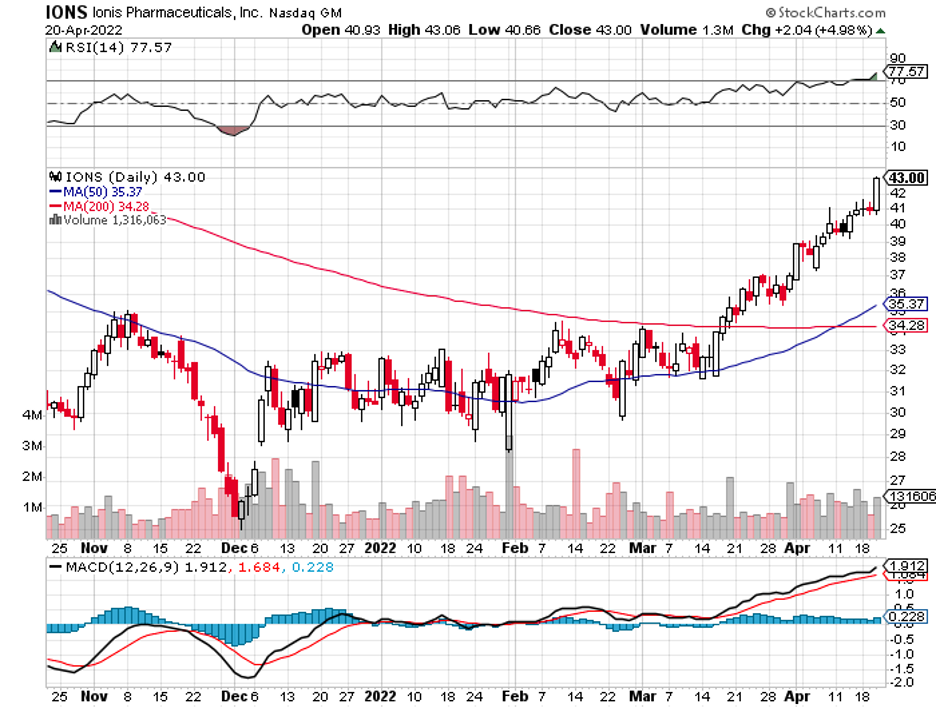
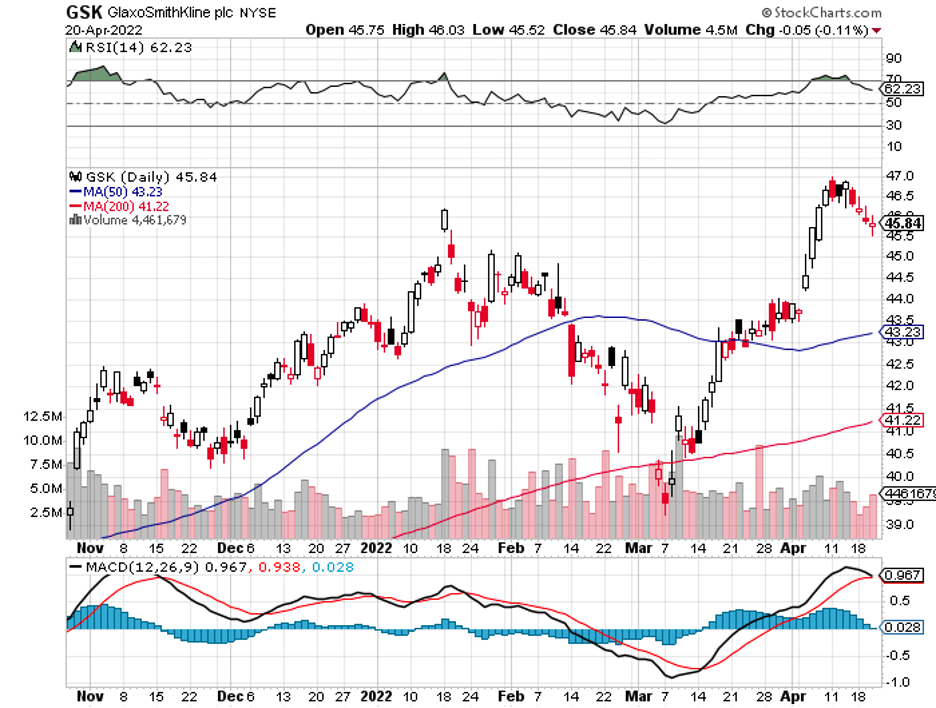
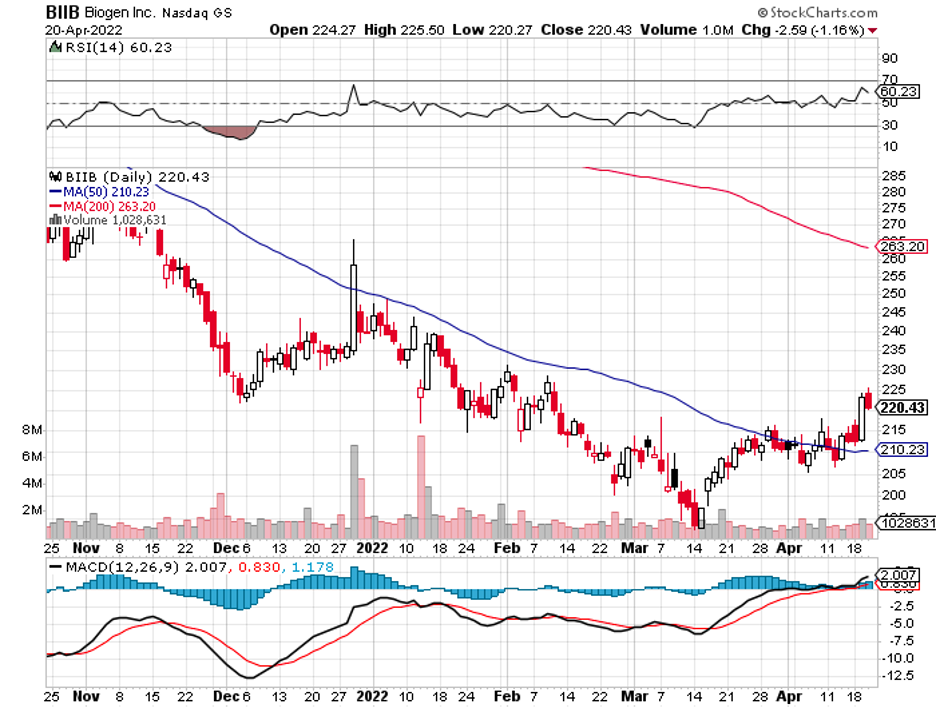
Another RNA-focused company, Ionis Pharmaceuticals (IONS), appears to be a key target as well, with the likes of GlaxoSmithKline (GSK), Bayer, and even Biogen (BIIB) waiting for an opportunity to pounce.
After all, acquisitions form an integral lifeline of the biotech world. Huge businesses with the resources swoop up promising buyout candidates to bolster their own pipelines.
However, M&A isn’t the only option for biotechs. There’s also the path where they can seek companies with similar focus and consolidate to become larger and more competitive entities.
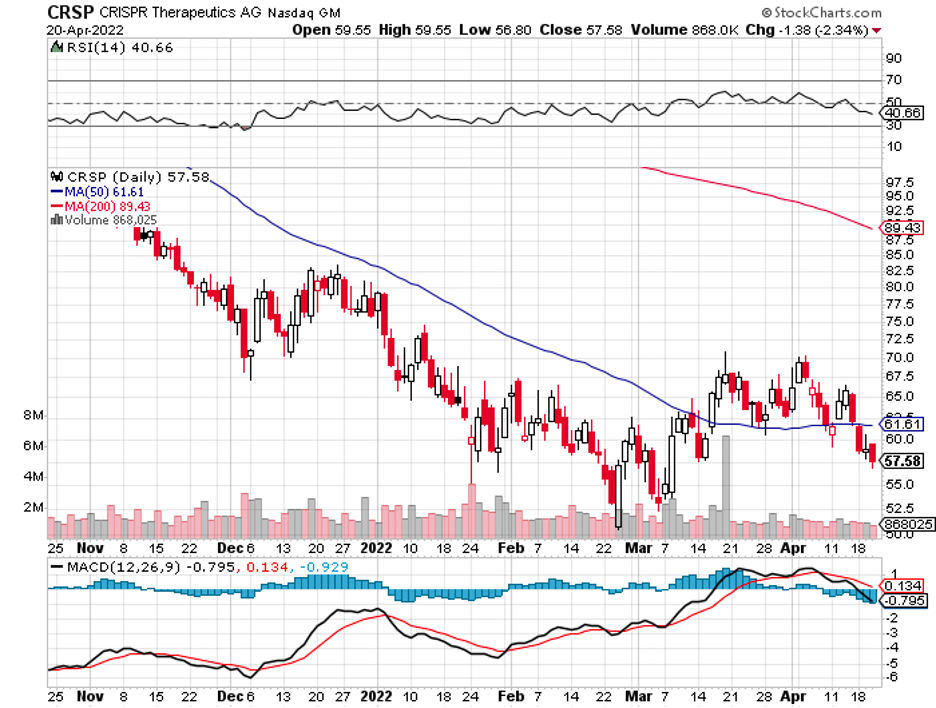
This has been the expected plan for CRISPR Therapeutics (CRSP) for a long time. Hence, it is no surprise if other biotechs with their own groundbreaking technologies decide to follow the same route.
Overall, the biotech industry is booming amid its recent struggles with the market.
The faster growth rate of companies can be attributed to more investors seeing the industry's potential and, of course, better access to technology and scientific advancement.
Moreover, the world has become more interested in the biotech world and what the industry can offer due to the pandemic.
COVID-19 has shone a light on this sector following the quick and effective results of the vaccines and treatments.
That is, people have finally caught on to the idea that there is an incredible opportunity in biotech.
While a correction is to be expected at some point, the critical thing to bear in mind is that great ideas will always generate funding no matter what.