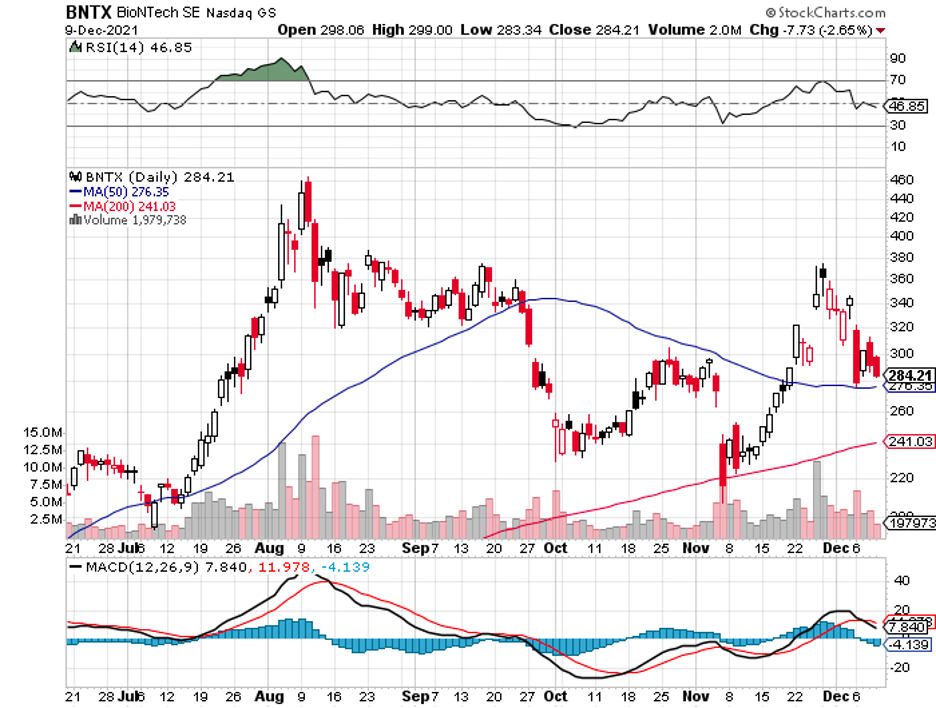
Almost everything that could go right has gone right for BioNTech so far.
Its COVID-19 vaccine with Pfizer (PFE), Comirnaty, has been breaking records left and right, and more and more approvals in other countries are piling up.
Needless to say, BioNTech has transformed into one of the most profitable biotechnology companies with a rapidly growing cash stockpile.
Now, the company is up for another challenge: the Omicron variant.
Although BioNTech and even Moderna (MRNA) insist that they offer more than COVID vaccines, the reality is that their pipelines still have not reached the stage where they can generate as much revenue.
Hence, it is no surprise that their share prices have climbed since discovering the Omicron strain.
The emergence of this new mutation sparked another competition among COVID-19 vaccine developers, specifically in the mRNA segment dominated by BioNTech and Moderna.
Since news broke about the Omicron variant, these companies have been racing to come up with the most effective vaccine against it.
BioNTech holds a competitive advantage between the two since the company reportedly has been working with Pfizer on a vaccine candidate for this type of situation months before the discovery.
In comparison, Moderna has yet to determine where their candidate stands in terms of fighting off the new variant.
The same can be said about other vaccine developers like AstraZeneca (AZN) and Johnson & Johnson (JNJ).
What happens to their efforts if the Omicron variant turns out to be less dangerous and possibly closer to the common flu?
In this case, the vaccine developers would most likely boost the prices of their products 10-fold because then they’d end up with fewer orders to private customers instead of sealing agreements with governments.
The flu vaccine market is worth roughly $8 billion annually, while the COVID vaccination market is projected to bring in approximately $25 billion each year in the post-pandemic period.
Either way, this situation could offer speculative investors a solid stream of price catalysts.
The uncertainty will result in a higher valuation for BioNTech in the short term because the company has already proven its ability to deliver an effective vaccine within a short period.
Prior to its COVID work, BioNTech was actually known as one of the “Big 3” and a pioneer in the mRNA world. At that time, it shared this title with Moderna and CureVac (CVAC).
Since then, the segment has grown, and new challengers have joined the mRNA industry.
Some of the promising ones include China’s Abogen Biosciences, which managed to raise over $700 million in funding for its own mRNA COVID vaccine, and of course, Sanofi (SNY), which splurged in a $3.2 billion acquisition of Translate Bio to access the latter’s mRNA pipeline for cystic fibrosis and several genetic conditions.
Meanwhile, BioNTech has retained its focus on cancer, with 16 of the 18 programs targeting oncology in its Phase 1 pipeline.
If BioNTech successfully develops an mRNA treatment for cancer, they’ll be breaking into a massive and lucrative market.
By 2024, the market for cancer treatments is projected to grow and reach over $200 billion.
Apart from its work on oncology therapies, BioNTech is also known for its infectious disease pipeline, including vaccines for HIV, malaria, and tuberculosis. It’s also collaborating with Pfizer on 2 influenza vaccines.
By the end of 2021, BioNTech is anticipated to release 5 updates on its vaccine trials involving solid tumors that target head and neck cancer, melanoma, and colorectal cancer.
Other than Pfizer, the company has been working with Regeneron (REGN), Genentech, Merck (MRK), Bristol Myers Squibb (BMY), and Sanofi.
In terms of performance so far, BioNTech has raked in $15.2 billion in revenues for the first three quarters of 2021, with full-year earnings expected to reach $18.1 to $19.2 billion.
Overall, I view BioNTech as a long-term investment.
While many still see it as a pure COVID play, this German company is increasingly starting to act more and more like the Big Pharma organizations.
It’s realistically expecting that its profit-generating asset, Comirnaty, may not have a very long shelf life. Therefore, it understands the necessity to come up with new products to sustain its current valuation over the longer term.