Building a retirement portfolio is different from when you’re aggressively playing the market. With this, you’d want something with less risk and more stability. A healthy helping of income definitely wouldn’t hurt either.
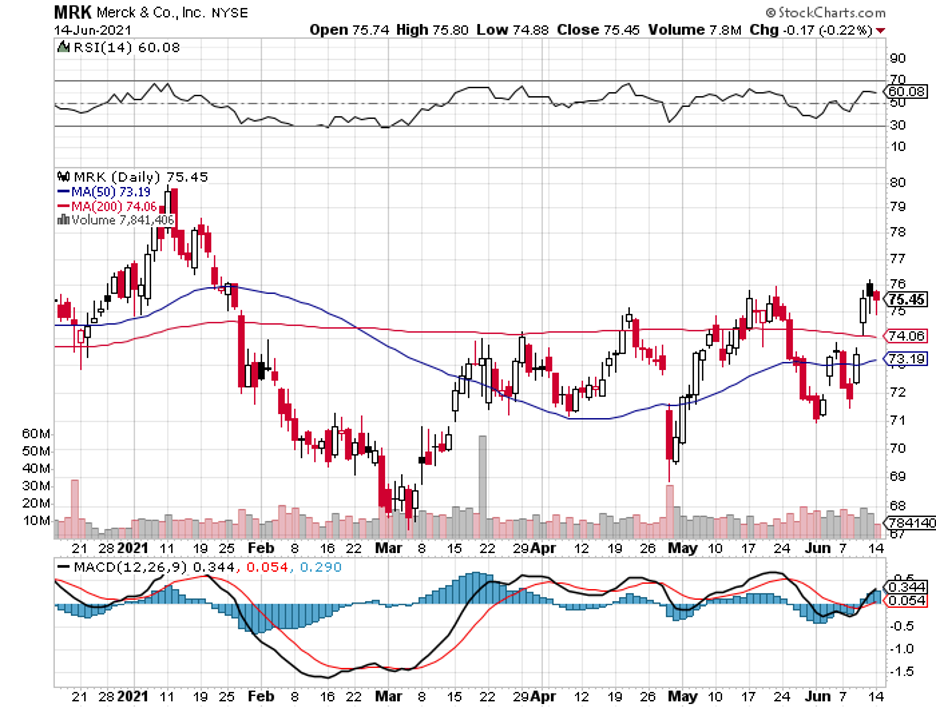
Taking these into consideration, a particular stock that offers a well-balanced mix of income and capital appreciation comes to mind: Merck (MRK).
The biggest news for Merck recently is its $1.2 billion deal with the US government involving its experimental COVID-19 antiviral.
The treatment, called Molnupiravir, is expected to cost about $700 per course, putting the total of the order from the US to 1.7 million courses.
This is just the beginning though. According to Merck and its partner, Ridgeback Biotherapeutics, they can produce at least 10 million courses of Molnupiravir by the end of 2021.
If we use the same pricing as the US, then we can expect approximately $7.1 billion in sales for Molnupiravir alone this year.
Still, the $1.2 billion deal with the US is already a massive win for Merck as experts initially estimated that Molnupiravir sales would only reach $25 million this year.
What makes Molnupiravir unique and more advantageous than its competitors is that the drug is taken orally.
The convenience alone easily edges out the other monoclonal antibody therapies from the likes of Regeneron (REGN), GlaxoSmithKline (GSK), and Eli Lilly (LLY)—all of which need to be administered intravenously.
If Molnupiravir does gain emergency use authorization from the FDA, its sole competitor in the market today is Veklury from Gilead Sciences (GILD).
To offer an idea on the size of the market for this treatment, Gilead recorded $2.8 billion in sales of Veklury in 2020. This figure is even projected to go up to $2.9 billion for this year.
Apart from its COVID-19 program, Merck has always been a favorite among value investors.
It’s a great dividend stock and has gained a reputable name in the industry as being one of the biggest and oldest companies in this field.
It’s also the force behind blockbuster treatments like the top-selling cancer drug Keytruda, HPV vaccine Gardasil, and of course, the diabetes medication Januvia.
In fact, Keytruda is estimated to become the No. 1 selling drug in the world by 2023—an achievement that Merck has lots of time to capitalize on considering that the treatment’s patent exclusivity lasts until 2028.
Keytruda is a key revenue generator for Merck, with the cancer drug showing off a 19% jump to reach $3.9 billion in sales in the first quarter of 2021.
This puts it on track to rake in roughly $16 billion in sales for this year, showcasing an 11% increase from 2020.
By 2026, Keytruda is estimated to generate $24.32 billion in sales annually.
Apart from Keytruda, Merck has been boosting its pipeline as well. For example, Bridion, one of its newer drugs, raked in $1.2 billion in sales in the first quarter, which is up 6% year-over-year.
Looking at its history, Merck has repeatedly shown that it can compete aggressively in the biopharmaceutical industry.
In 2020, the company still managed to generate $48 billion in sales despite the pandemic, with an earnings per share of $5.94—a value that’s 65% stronger than it was just five years ago.
Its strong profit growth and promising pipeline programs have allowed the company to boost its dividend payout at an impressive 7.1% pace over the past years.
This is a performance that most blue-chip companies, regardless of their size and market cap, struggle to keep up with.
Merck isn’t as exciting as the other stocks in the biotechnology and healthcare market, but that’s a comforting thought for investors who are on the lookout for a stable business.
Although Merck stock is not dirt cheap, I think it’s attractive for those who have extra cash or are hesitant to roll the dice on more volatile companies today.