The ongoing seesaw fights in the stock market are causing too much drama that cunning investors can—and definitely should—steer clear from.
Instead of fretting over speculative and risky investments, it’s better off staying with tested and proven big-name companies that will remain solid buys for the years to come and continue to deliver positive results despite periods of uncertainty.
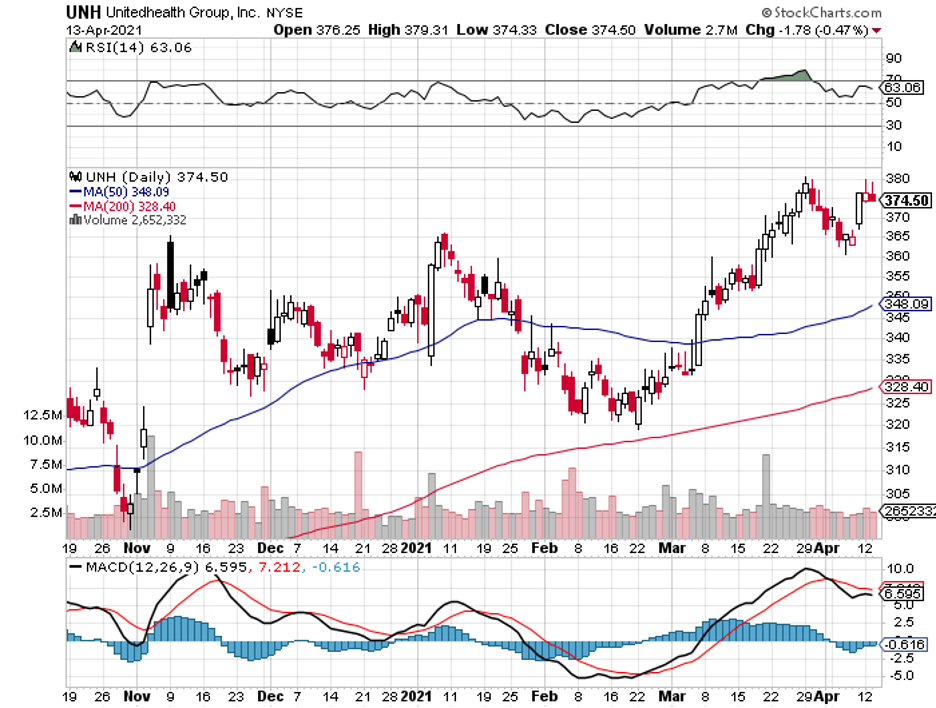
Among the huge names in the healthcare industry, United Health (UNH) is a strong contender that meets the criteria.
After almost a decade of delivering high returns, which shows off fast-rising earnings expectations, it’s interesting to see that the market has recently backed away from this blue-chip stock.
Nonetheless, the market’s skittishness offers an opening for investors looking to buy in the dip a company that pays dividends and promises to steadily boost your savings in the years to come.
Founded way back in 1974, UNH has become a top name in the healthcare industry, landing in seventh place on the Fortune 500 list.
To date, UNH has four main divisions handling its over 50 million members both in the US and across the globe: its private health insurance business UnitedHealthcare, its pharmacy benefits segment OptumRx, its healthcare services provider branch OptumHealth, and its analytics platform OptumInsight.
UNH has a market capitalization of $354 billion.
In comparison, the closest competitor is Anthem (ANTM), with $87.93 billion. In terms of market cap, the two are followed by Cigna (CI) with $85 billion, Humana (HUM) with $53.81 billion, and Centene (CNC) with $36.19 billion.
Amid the financial crisis brought about by the pandemic, UNH still reported a 6.2% jump in its revenue in 2020 to reach $257.1 billion.
The company’s most prominent growth driver is its Optum line, and UNH is making sure that this division continues to grow.
One of the most indicative moves towards that direction is UNH’s $7.8 billion acquisition announcement of technology and data analytics company Change Healthcare (CHNG), which should be completed by the second quarter of 2021.
In the agreement, UNH is offering Change Healthcare $25.75 for its shares, representing a premium of roughly 41% above the latter’s stock price.
UNH plans to merge Change Healthcare’s operation into OptumInsight, which currently handles hospital systems health plans, life sciences companies, and even governments.
In the first nine months of 2020 alone, OptumInsight generated over $1.9 billion, contributing to roughly 11% of UNH’s total bottom line.
The combination of these two will all but guarantee that UNH’s possession of the biggest and most powerful platform in the entire healthcare industry, with the acquisition projected to add approximately $470 million to the company’s adjusted earnings in 2022.
The decision to acquire Change Healthcare is part of the string of M&A deals executed by UNH to stay ahead of the game.
In 2019, it bought two companies to expand its operations: the DaVita Medical Group for $4.3 billion and Equian for $3.2 billion. Prior to these, UNH splurged on a $12.8 billion acquisition of Catamaran in 2015.
For 2021, UNH projects its earnings to increase, estimating its per-share profits to be somewhere between $16.90 and $17.40—and that estimate already took into consideration the headwinds involving COVID-19 that could still weigh on the company’s bottom line.
While this may appear optimistic, the truth is, generating strong results isn’t a novel accomplishment for UNH.
In the past three years, the company reported a net income of $10 billion or higher, with net margins recorded at 5% above its revenue.
Over the course of the last 12 months, UNH stock has climbed 24%, beating the S&P 500, which rose 18% during the same period. It also offers a decent dividend of 1.5%, which is admittedly slightly lower than the S&P 500 average at 1.6%.
Overall, UNH is a safe stock that you will not have to anxiously watch over and can hold in your portfolio for years.
More importantly, this company remains undervalued and still shows a lot of room for growth.
So if you’re a value investor looking with an interest in the insurance and healthcare services industry, then this market leader is a sustainable addition to your portfolio.