CRISPR technology, long heralded as a game-changer in genomics, stands on the brink of a major leap forward. For years, its potential has simmered, but now, it's poised to ignite, promising scientific breakthroughs and significant investment opportunities.
Several pioneering companies employing CRISPR for editing human genomes are at the forefront of this revolution. Their goal? To treat, and potentially cure, a range of genetic diseases. The approaches are twofold: ex vivo, where genes are edited outside the body, and in vivo, with modifications made directly within the body.
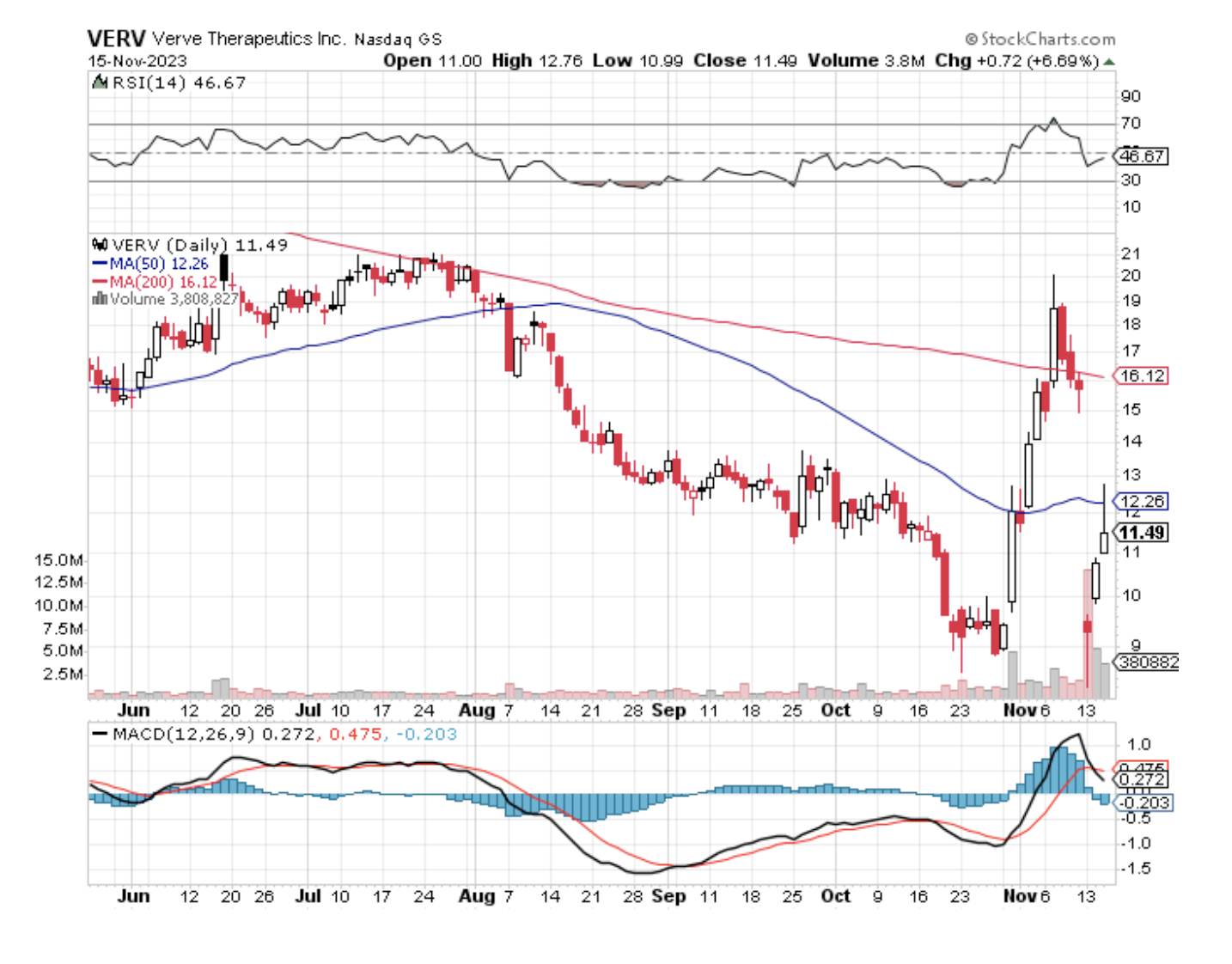
Investing in CRISPR gene-editing stocks, however, is not for the faint-hearted. These stocks are characterized by high risk and volatility, demanding a specific investor profile: one that is aggressive and comfortable with risk. For such investors, a company worth considering is Verve Therapeutics (VERV).
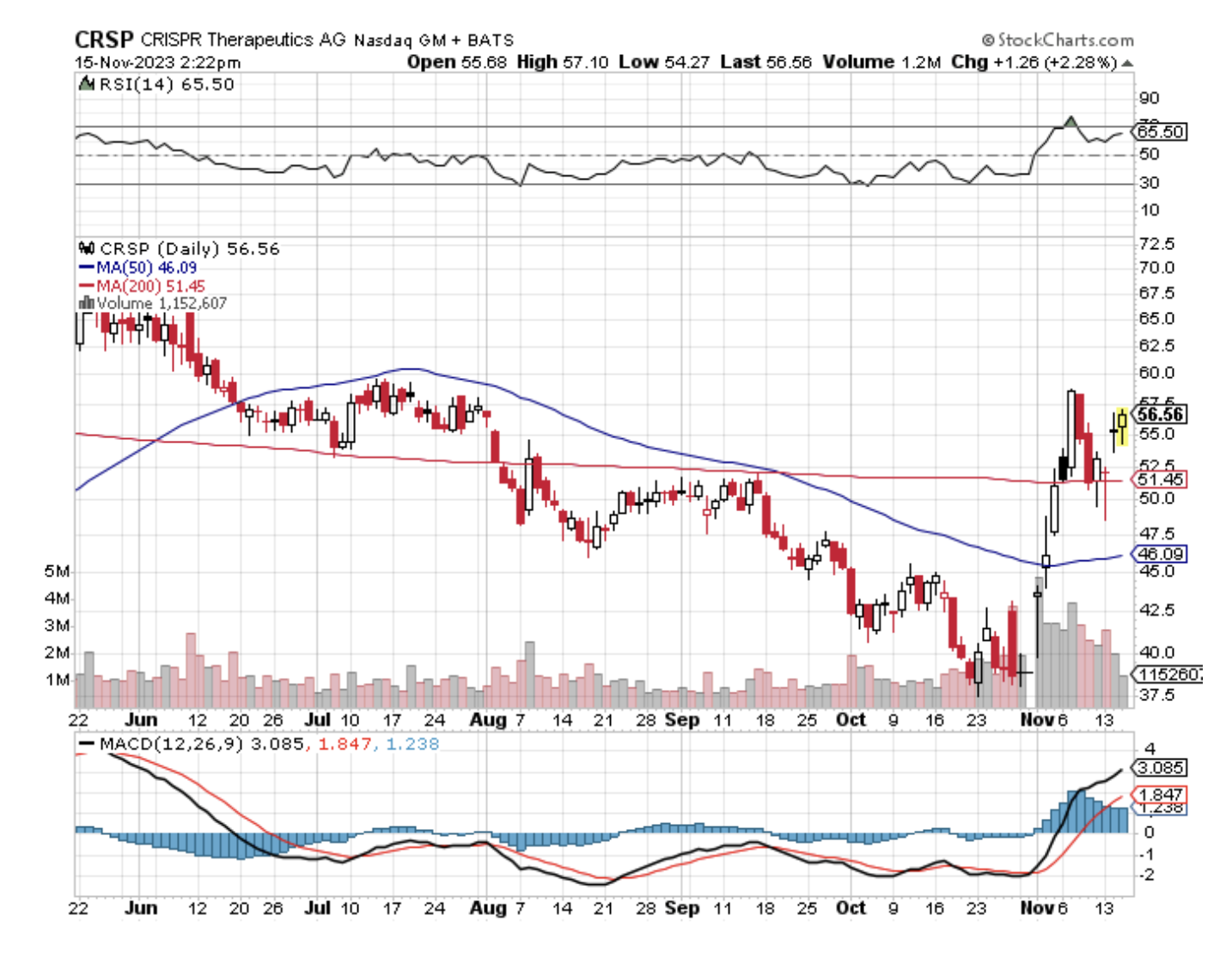
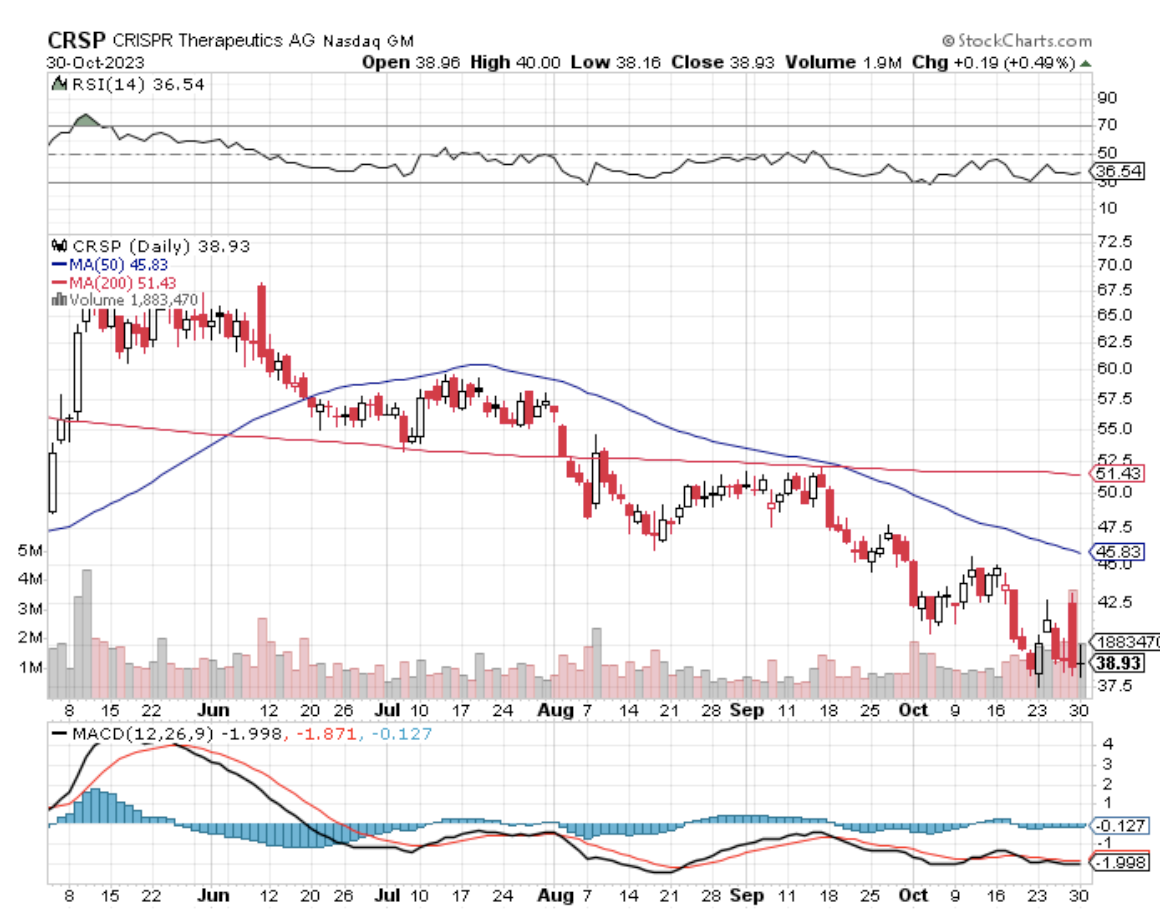
Verve stands out, partly due to its relatively modest size with a market capitalization of $732 million. This contrasts sharply with industry peers like CRISPR Therapeutics (CRSP) and Beam Therapeutics (BEAM), valued at $4.47 billion and over $2 billion, respectively. The reason behind Verve's smaller scale is its developmental stage, which lags behind its counterparts.
Established in 2018, Verve has been hailed as a potential leader in next-generation gene therapy, particularly base editing.
You can think of base editing as using a fine-tipped pen to precisely change just one letter in the DNA sequence, without cutting the DNA strand.
In our DNA, there are four "letters" (bases) – A, T, C, and G. Base editing lets scientists directly convert one letter to another (like changing an 'A' to a 'G') without cutting the DNA. This is like fixing a typo in a sentence by carefully erasing one letter and writing in the correct one.
This method is often more precise than CRISPR and less likely to introduce errors because it doesn't involve cutting the DNA strand.
Verve has capitalized on this technology, in-licensed from base-editing pioneer Beam Therapeutics. The company's flagship candidate, VERVE-101, targets heterozygous familial hypercholesterolemia (HeFH), a rare cholesterol disorder.
Needless to say, the stakes are high. The HeFH market is projected to balloon to nearly $60 billion by 2033, positioning VERVE-101 as a potential one-time functional cure and a standard of care in this lucrative market.
Recently, Verve announced that there was a substantial reduction in patients' high cholesterol levels in the first human test of base editing. Despite this, the stock experienced a sharp 41% drop, possibly a misinterpretation of the positive news in an unfriendly biotech market.
The data presented showed Verve's treatment leading to a 40%-55% decrease in harmful LDL cholesterol levels in patients with genetically high cholesterol levels. Verve's approach targets and inactivates the defective gene responsible for high cholesterol levels.
The treatment, however, faced challenges. Two of the Verve-101 trial participants suffered heart attacks, one of which was fatal.
It's crucial to note that the trial specifically included older patients with advanced heart disease, who were already at a heightened risk for cardiac events. The overall safety measures in the study were satisfactory, though, so the FDA has since authorized an expansion of the Phase 1 trial.
Notably, Eli Lilly (LLY) reviewed the trial's results before deciding to buy an option to partner on the Verve treatment. Lilly's decision on teaming up on the cholesterol treatment is expected next year, following the completion of Phase 1 trials.
Additionally, Verve plans to initiate trials for another base-edited therapy, VERVE-102, in the first half of 2024, potentially offering enhanced patient outcomes.
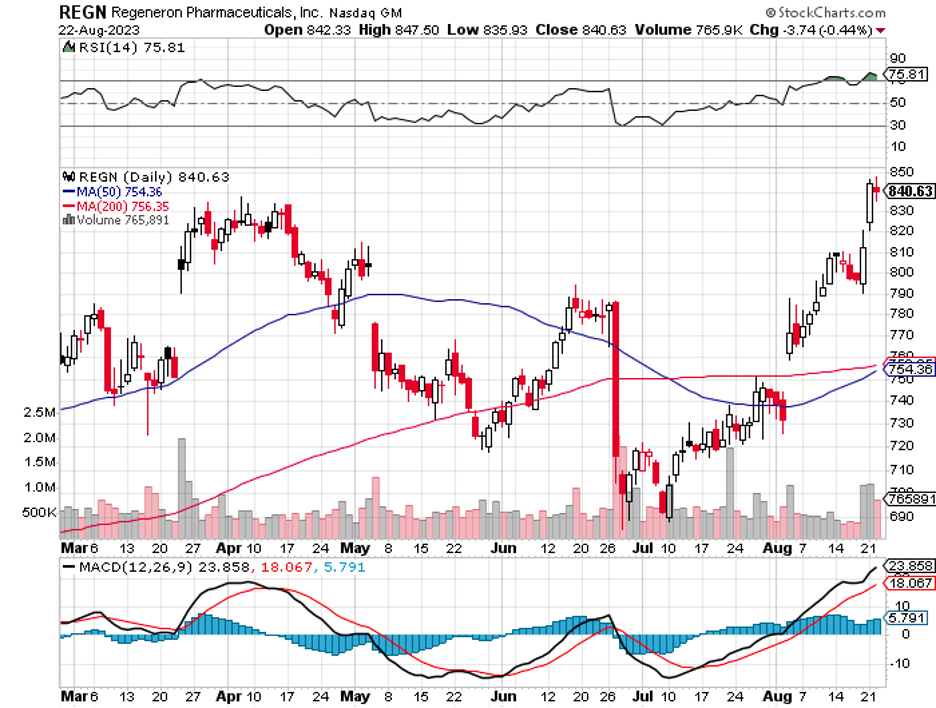
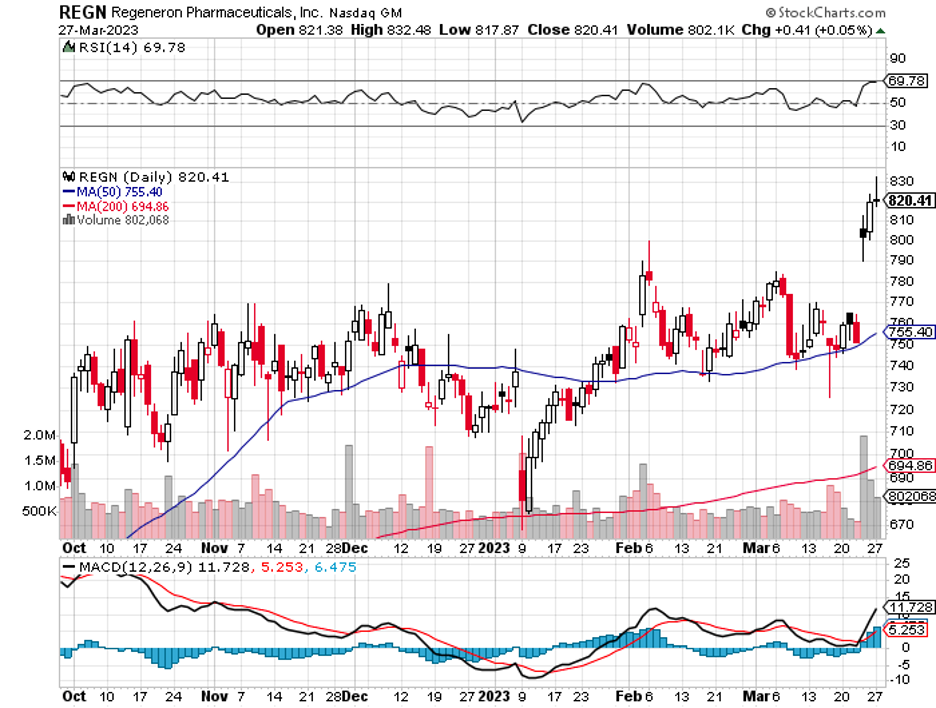
Verve’s trial results match that seen with established medications such as Novartis' Leqvio (NVS), Amgen's Repatha (AMGN), and Regeneron Pharmaceuticals' Praluent (REGN), which are all approved long-term drug therapies.
However, despite the availability of statins and new treatments, a significant portion of these patients fail to maintain healthy cholesterol levels due to cost, treatment adherence issues, or inconsistent healthcare access.
This is where the biotech company’s solution shines. Verve's ultimate goal is to develop a one-and-done treatment to lower cholesterol in the 50 million adults at risk for cardiovascular disease.
While Verve remains a preclinical-stage biotech, its prospects are promising. Its market cap, though modest compared to the commercial opportunity of a functional cure for HeFH, hints at significant growth potential.
With Lilly's track record in developing drugs for underserved conditions, Verve emerges as a compelling investment for those with a high tolerance for risk and an eye on future biotech breakthroughs. I suggest you put this stock on your watchlist.