The routine medical check-ups we have today are primarily based on physical exams that were developed way back in the 1820s, utilizing tools that haven’t been upgraded for over a century.
More alarmingly, all we go through is a “comprehensive” health check once every year, offering us just a snapshot of what’s truly going on in our bodies.
If anything, we monitor the releases of new software for our phones and laptops more than we pay attention to our own bodies.
As we’ve proven with the COVID-19 pandemic, so much can happen in a year
Truth be told, our bodies can deteriorate at lightning speed and without any warning. That’s why it’s terrifying to think that we’re not doing as much to monitor our health.
So, what can we do to change this? How can we be more proactive when it comes to our health?
The COVID-19 pandemic has brought many changes into our lives, and this is one of the biggest transformations it has done: an exponential spike in demand for telehealth services.
One of the major issues between patients and doctors at the height of the pandemic was how to go through the physical exams without actual physical contact.
Clearly, it’s not possible to hear a heart murmur or irregular breathing over a video call.
This is where a lot of innovative companies come in.
For a more specialized exam, HD Medical released a credit card-sized device called HealthyU.
Patients simply touch it with their finger, and the device can instantaneously measure their heart rate and sounds, temperature, and even oxygen saturation.
All these data would then be sent to their doctors or health providers in real-time.
HealthyU also has a remote EKG, which effectively allows it to serve as a portable roadmap to a patient’s heart health and helps doctors monitor for signs of heart attacks and arrhythmias.
For example, there’s this handheld exam kit called Tyto that patients can use to perform their own guided medical exams.
This palm-sized gadget is linked to an app, so your doctor can monitor you remotely.
Patients suffering from a sore throat can use Tyto’s camera to let the doctors see the back of their throats, while those struggling from chest pains can easily use the stethoscope to help their physicians listen to their lungs and hearts.
And these are just for physical exams. There are more advancements in health monitoring, and this is where wearable technology comes in.
Wearable technology is considered one of the most promising growth drivers, largely due to the health sector.
The market size for this segment is estimated to rise from $116.2 billion in 2021 to $265.4 billion by 2026, showing off an 18% CAGR growth within a 5-year period.
Applications for wearables have expanded to areas including medical surgery as well as internables and implantables or sensors, which can be fitted into our bodies to help doctors observe various health parameters.
It’s no wonder brands like Apple (AAPL) with Apple Watch, Google (GOOGL) with Fitbit, and Garmin (GRMN) have been working overtime to try to cover as much of the wearable health market as possible.
So far, these products provide extensive data ranging from calories burned to our heart rates.
Aside from them, there are other wearables in the market today that could change the landscape of the health industry.
One of them is the Oura Ring, which was first introduced in 2013.
Designed to be worn 24 hours a day, this device measures the bodily functions of the user. It gathers data through infrared light sensors that touch the finger arteries.
One of the most impressive things it can do is monitor your sleep movements to help determine early onset of some neurodegenerative diseases like Parkinson’s.
The information is all sent to the app, which users can access via their smartphones. The Oura Ring is somewhere between $299 and $999, depending on your preferences in style and color.
Although it’s yet to be a mainstream product, the Oura Ring was provided to NBA players when they resumed their season amid the COVID-19 pandemic.
The device was used to help the basketball stars monitor their health.
In fact, a joint study with the University of California San Francisco showed that the Oura Ring was able to help detect the common symptoms of COVID-19 three days earlier and with as high as 90% accuracy.
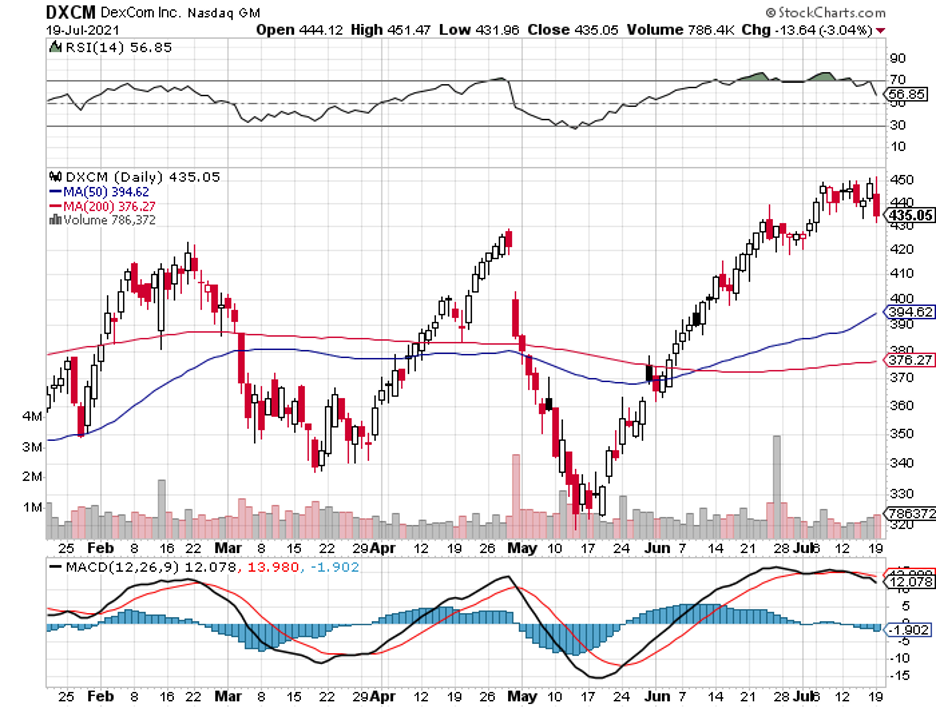
Another impressive health monitoring advancement covers the glucose monitoring product line of Dexcom (DXCM).
The primary goal of Dexcom is to take away the guesswork that comes with finger pricking.
By offering a wearable sensor, people with diabetes can easily and accurately monitor their glucose levels.
What’s even more convenient is that Dexcom’s wearable is available in practically all large pharmacies like CVS (CVS), Walgreens (WBA), and Rite Aid (RAD).
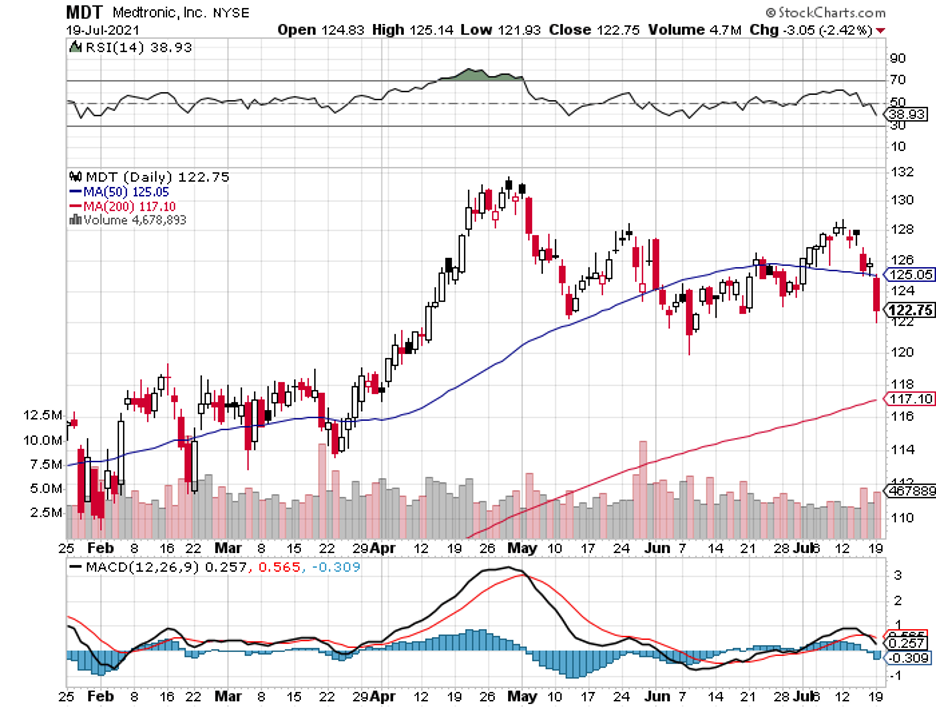
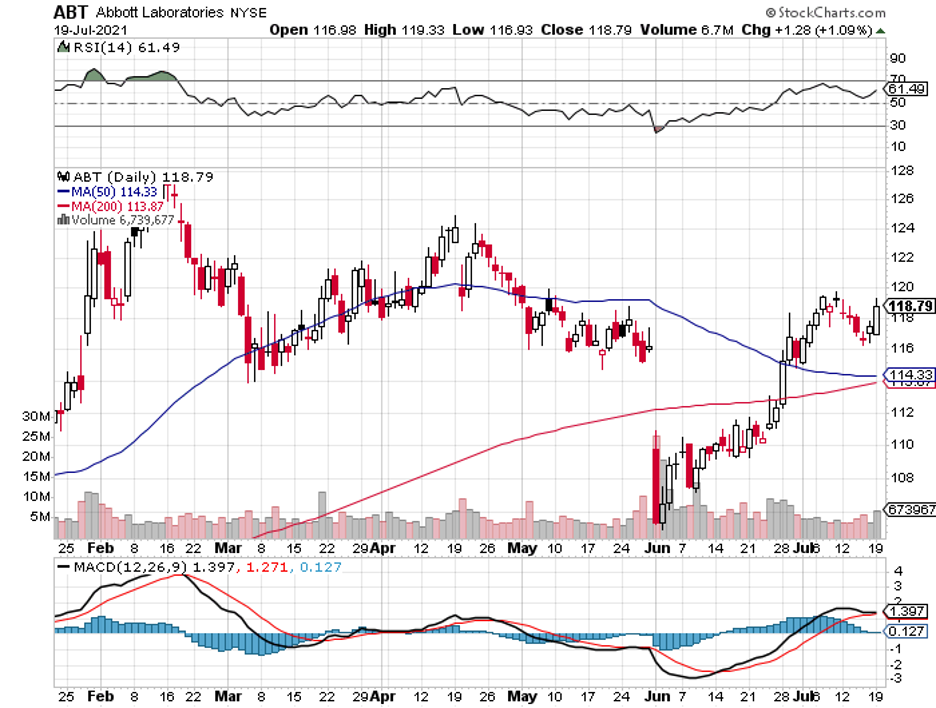
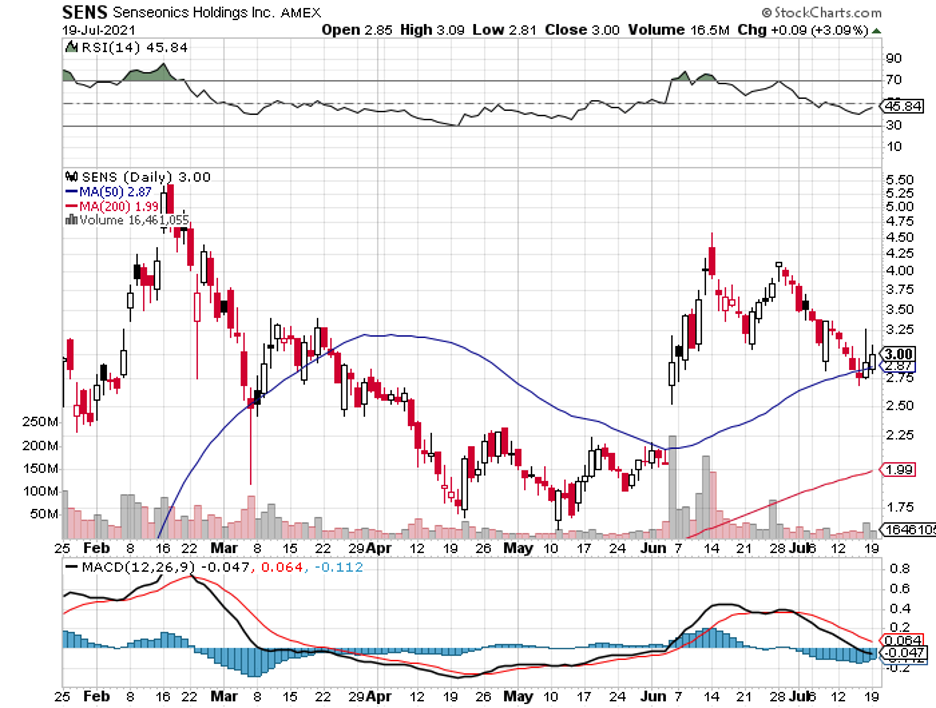
To date, Dexcom’s biggest competitors include Medtronic’s (MDT) Guardian Connect, Abbott’s (ABBT) Freestyle Libre, and Senseonics’ (SENS) Eversense.
These are only some of the emerging technologies that could help us improve the quality of our lives today, with thousands more expected to follow suit in the years to come.
For an endlessly advancing world with smartphones, supercomputers, smart homes, and even self-driving cars receiving software updates virtually every week, it’s absurd to think that we only allot a single check-in on our health annually.
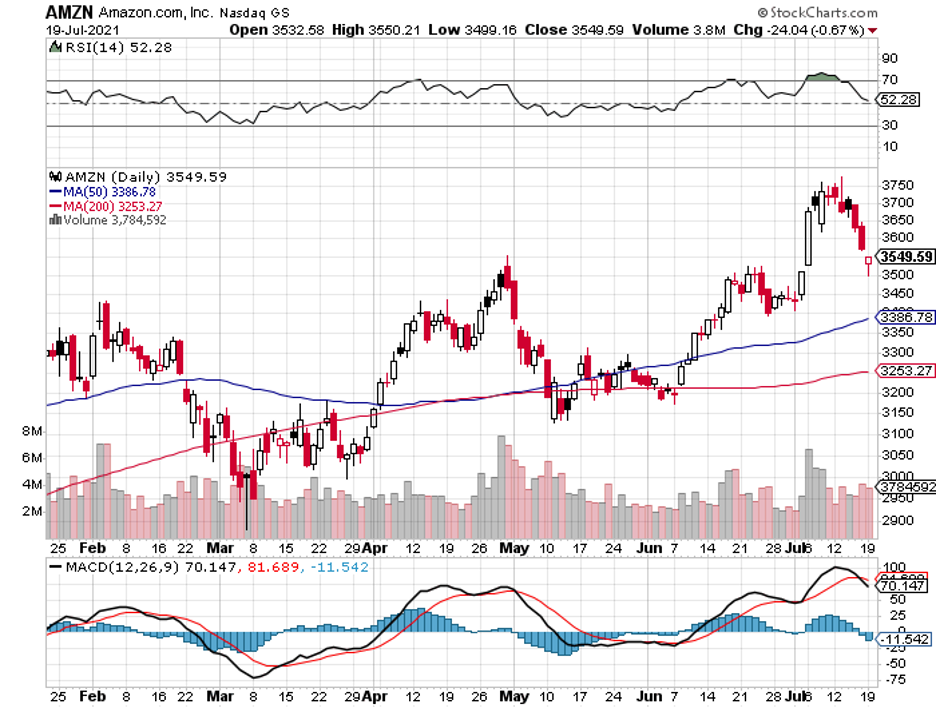
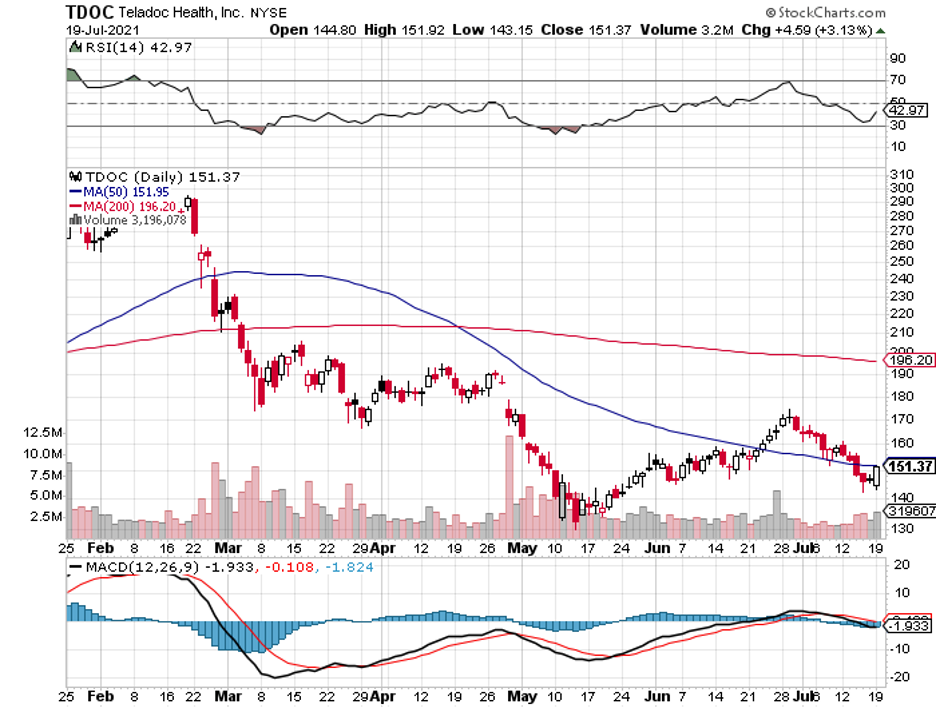
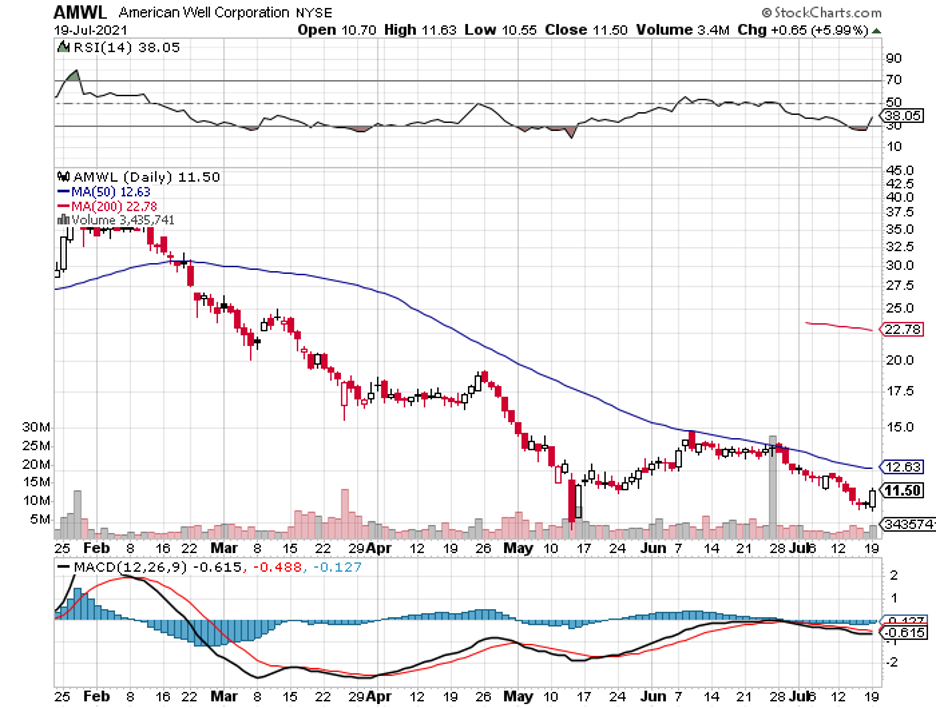
But with the advent of these technologies and the increasing popularity of telehealth services spearheaded by the likes of Teladoc (TDOC), Amwell (AMWL), and even Amazon (AMZN), it looks like we’re starting to finally pay more attention to our health.