Monopolies. In any industry, they’re typically called rule breakers.
For healthcare, these are rule-making stocks that ultimately rise to dominance that they eventually win government-sanctioned monopolies and establish massive networks.
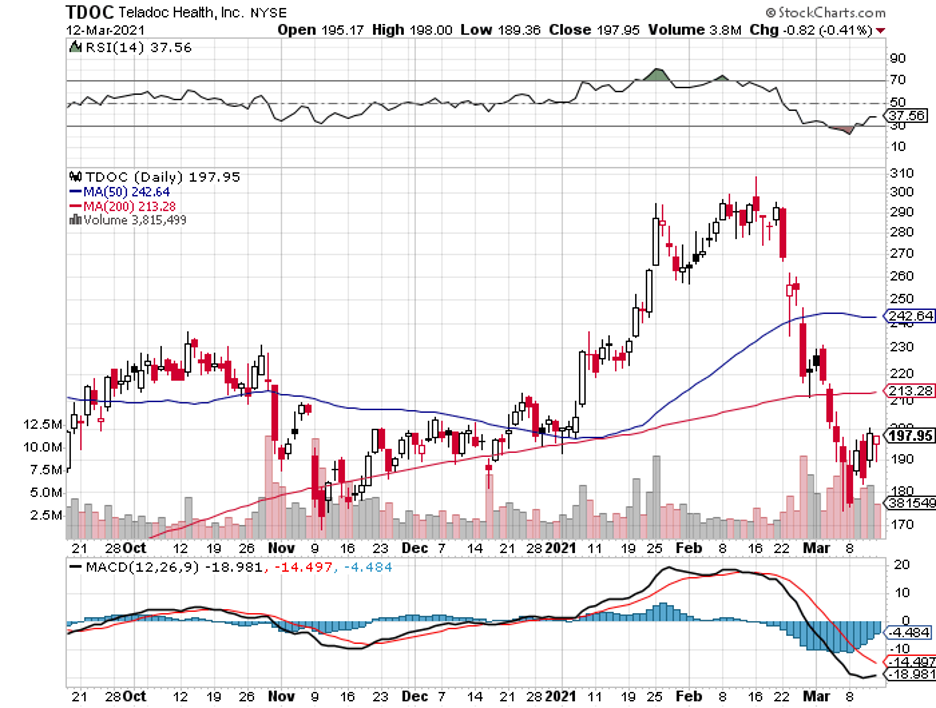
For biotechnology and healthcare investors who like to err on the side of caution but still want to take a dip on monopoly-like players, one stock stands out: Teladoc Health (TDOC).
Teladoc, which currently has a market capitalization of $30 billion, is one of those groundbreaking companies that use technology to disrupt the way we live.
The company’s potential is actually getting compared to the likes of other tech movers such as (SQ), Shopify (SHOP), Roku (ROKU), and Tesla (TSLA).
So far, Teladoc stock has gone up 144% over the past 12 months.
On a year-over-year basis, the total number of visits for Teladoc more than doubled from 4.14 million to reach a whopping 10.59 million. Even the international visits rose by 71%.
As expected, the COVID-19 pandemic served as a major boon to its already thriving business.
Although it wasn’t as popular at the time, the company’s operating model offered a myriad of benefits for the healthcare industry.
For one, telehealth visits are way more convenient for the patients. Since the visits won’t take as much time and effort from their end, the patients would be more motivated to regularly check in with their doctors.
In turn, the doctors would be able to offer higher-quality care since the cooperation of the patients means they can also monitor the symptoms and progress better.
Best of all, the patients are typically billed at cheaper rates compared to office visits.
I think the last one makes Teladoc an attractive winner in the eyes of practically all health insurers.
Teladoc is the biggest telehealth services provider in the United States, and one of the major steps that the company took to cement its reputation as a virtual health leader is its splashy $18.5 billion merger with Livongo in 2020.
If you haven’t heard of Livongo, this company was growing incredibly faster than Teladoc even before the merger.
Basically, Livongo collects copious amounts of information from patients. Using artificial intelligence, the company then sends personalized tips and reminders to their enrolled members with the goal to improve their overall quality of life.
Most of the patients suffer from chronic conditions, which means they would require regular nudges to ensure that they take the proper medications on time.
For example, some of Livongo’s users have diabetes. The company monitors them via wireless glucose meters, guiding the users to follow a positive lifestyle when their blood sugars begin to spike.
At the time of the merger, Livongo has already secured over 500,000 enrolled members for its diabetes platform.
This is impressive as it represents roughly 2% of the entire diabetes pool in the United States.
Aside from diabetes, Livongo has also been working on other chronic conditions like hypertension and weight management.
Considering that hypertension accounts for 7.6 million deaths per year worldwide and the extensive list of health problems associated with obesity, such as coronary heart disease and end-stage renal disease, I think Livongo has developed a highly sustainable business model that’s perfect for the “new normal.”
More importantly, the combination of Livongo and Teladoc will now allow the two companies to cross-sell their services to their users.
As for those who think that Teladoc is only a pandemic play, the company didn’t really need the pandemic for its business model to succeed.
Prior to COVID-19, its sales have been growing by an average of 75% per annum since 2013.
With its merger with Livongo, I think Teladoc has developed a stronger all-weather model for growth.
Teladoc is a rule maker and a first-mover, with the company moving the multi-trillion dollar healthcare industry to the internet.
At this point, it is the dominant name in the arena and the undisputed leader in telehealth. With everything it has to offer, I believe Teladoc is a long-term investing opportunity and it would be a good idea to buy on the dip.