It was a case of being in the right place at the right time.
BioNTech (BNTX) has always been focused on mRNA technology, so when Big Pharma player Pfizer (PFE) knocked on its doors for a collaboration, this up-and-coming biotech company was more than ready to go.
We all know what happened after that. BioNTech and Pfizer became the first to bring a COVID-19 vaccine to the public.
And just like how the pandemic changed the fortune of Moderna (MRNA), the COVID-19 situation also served as proof of concept of BioNTech’s technology.
Looking at BioNTech’s history and recent performance, I can see several reasons to buy the stock.
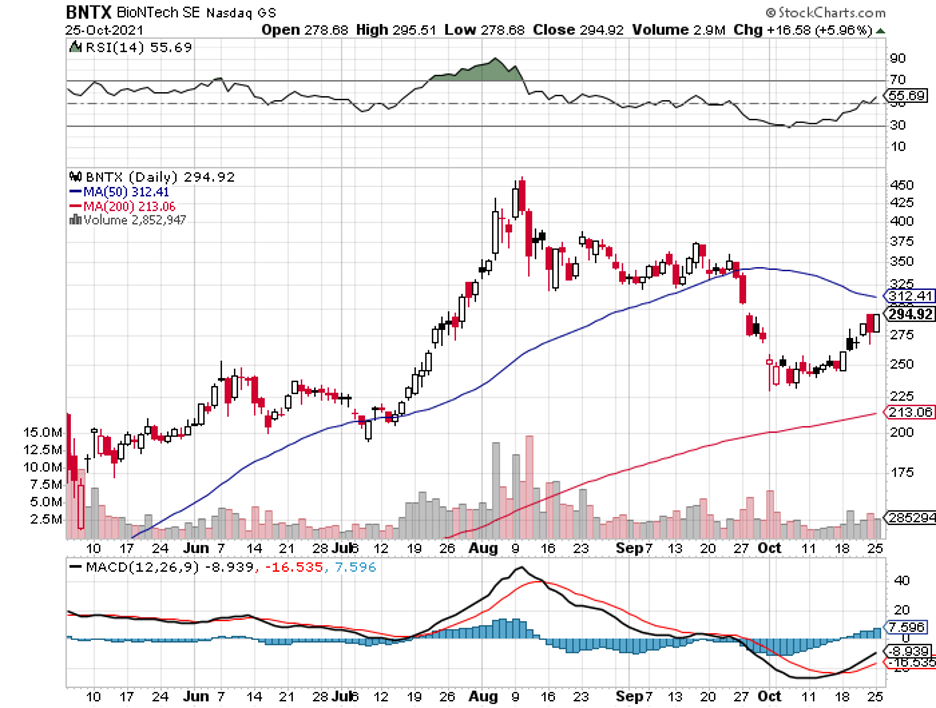
Short term, one of the primary reasons to buy BioNTech is obvious: its overwhelming success in creating a COVID-19 vaccine.
BioNTech expects approximately $18.4 billion in revenue from its COVID-19 vaccine in 2021.
In its second-quarter earnings report, BioNTech and Pfizer disclosed that they already crossed the 1 billion mark in terms of the vaccine doses delivered globally.
In fact, BioNTech’s revenues beat the projected $2.35 billion, with the company generating $6.4 billion in sales for the second quarter of 2021 alone.
This is an impressive jump from the $47.54 million it recorded during the same period in 2020.
Considering the consistently high demand for the BioNTech-Pfizer vaccine, it’s reasonable to expect that the momentum will be sustained.
To date, an additional 200 million doses have been ordered by the US government. This is on top of the 500 million doses it initially bought under the current supply agreement.
Meanwhile, the EU’s orders for 2021 reached 660 million doses plus 900 million more for 2022 to 2023.
Depending on the situation, another 900 million doses might be added to these initial agreements.
Just between the US and the EU, BioNTech has already received orders for over 1 billion doses of COVID-19 vaccines for 2022 onward—a number that’s widely expected to go up when other nations place their orders as well.
So far, the two companies have sealed an agreement with a South African biopharmaceutical company, Biovac, to collaborate on the manufacture and distribution of the vaccine across the 55 member states of the African Union.
As for the South American area, the partners have recently signed a deal with a Brazilian biopharma company, Eurofarma Laboratorios, to cover the Latin American regions.
Moving with the long-term reasons to invest in BioNTech, one of the most convincing aspects is the company’s promising pipeline.
BioNTech is realistic enough that the demand for its COVID-19 vaccine will eventually plateau. That has been the expectation since the beginning, which is why the company has been leveraging the incredible cash flow through expanding its pipeline.
Actually, BioNTech is allocating roughly $1.05 billion for R&D expenses in 2021.
Some of the segments that BioNTech has been working on are regenerative treatments and products for infectious diseases, inflammatory conditions, and allergies.
The company is also developing potential cancer therapies. After all, curing cancer is considered the Holy Grail of mRNA-centered companies—an achievement that would undoubtedly catapult BioNTech’s stock to the top of the Big Pharma list.
One of the telltale indicators of BioNTech’s plan to focus on oncology treatments is its July 2021 acquisition of Kite’s R&D platform on TCR Cell solid tumor neoantigen T-cell receptor (TCR) along with its manufacturing plant in Maryland.
The driving force behind that deal is BioNTech’s desire to become a first-mover in the cell therapy space.
Basically, the company added ammunition to its pipeline to come up with individualized cancer therapies.
BioNTech also has a couple of mRNA-based solutions queued for Phase 2 trials this year.
One is FixVac BNT111, which is developed for melanoma and a collaborative effort with Regeneron (REGN). This candidate has shown promising results, with the possibility of being available for use to over 90% of melanoma patients.
Others include FixVac BNT113, which targets head and neck cancer, and FixVac BNT112 for prostate cancer.
Another promising candidate is its cancer vaccine, INeST BNT122, which BioNTech is working on with Genentech.
Apart from these, BioNTech is also looking at developing treatments for infectious diseases as another potential long-term growth pillar—a direction taken by its biggest competitor in mRNA-based solutions, Moderna.
Checking its pipeline, it looks like BioNTech plans to target malaria as its first project. It also has candidates for HIV, tuberculosis, and influenza.
BioNTech’s goal is to launch the first-ever mRNA vaccine against malaria. If all goes according to plan, the company plans to conduct clinical trials by 2022.
Meanwhile, its influenza vaccine program, which faces serious competition against Sanofi (SNY) and Novavax (NVAX), will be another collaborative work with Pfizer. The two companies plan to initiate human trials before the end of 2021.
Pretty much like Moderna, I look at BioNTech as a long-term play. Investing in this company requires patience and belief in the burgeoning mRNA space.
Overall, I think BioNTech is a safe bet for investors looking to dip their toes in the rapidly expanding mRNA world.