Another day. Another COVID-19 vaccine could be out on the market.
Although hundreds of millions of individuals across the globe have already received their shots from approved vaccines of Pfizer (PFE) - BioNTech (BNTX), Moderna (MRNA), and Johnson & Johnson (JNJ), there are still several COVID-19 vaccine candidates undergoing late-stage testing.
This is important news for the companies.
After all, the COVID-19 vaccine market is projected to become a fundamental driver of share price growth.
From what we know about the virus so far, there is a huge possibility that people will need to be vaccinated annually at the very least.
If that’s the case, then the demand for COVID-19 vaccines yearly would reach roughly 8 to 10 billion doses. This could translate to sales between $80 billion and $100 billion.
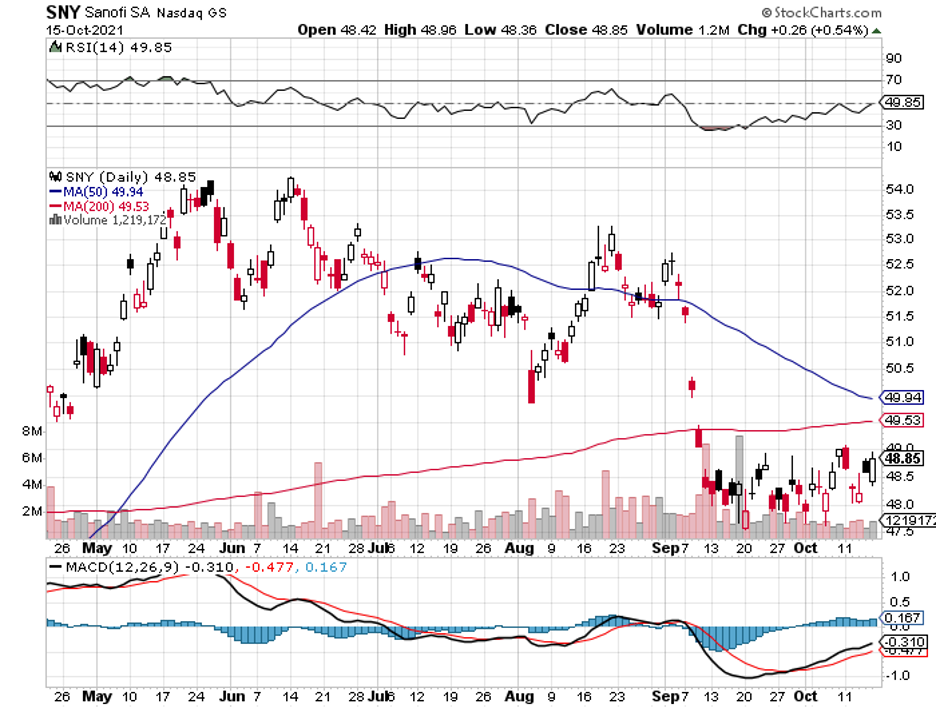
The latest to potentially join the list is the joint candidate of Sanofi (SNY) and GlaxoSmithKline (GSK), which is anticipated for approval by the fourth quarter of 2021.
While Sanofi and GSK are practically a year behind their competitors, the high efficacy of their vaccine candidate—roughly 95% to 100%—makes them potential frontrunners in the near future.
Given that we can expect many competitors to enter the fray in the coming months, we can conservatively assume that Sanofi takes at least 3% to 5% of the market.
That would generate approximately $2.4 billion to $5 billion in annual revenue.
Since Sanofi holds a number of competitive advantages, such as solid experience in manufacturing and development and a strong geographical presence across critical markets, the company can ease out the competition.
Riding the momentum of the COVID-19 vaccines, particularly the mRNA candidates, Sanofi has been working with Seqirus to develop flu vaccines as well.
As the world struggles to deal with the effects of COVID-19, the more common flu isn’t receiving that much attention these days.
However, the impact of this disease worldwide is shocking: 3 million to 5 million people suffer from severe cases annually, with up to 650,000 individuals dying from the flu.
More alarmingly, a new flu strain spreads from animals and causes a pandemic every few decades.
The death toll associated with the flu becomes even more staggering when you think about the fact that we’ve been working on vaccines to get rid of its for eight decades now.
Despite the ongoing and long-term efforts, all flu vaccines available in the market are mediocre at best.
In fact, a flu shot is only effective within a single flu season. Moreover, its effectiveness is only within the range of 40% and 60%. There were even years when the number was as low as 10%.
Now, though, we might have a better shot at developing more effective flu vaccines thanks to the emergence of mRNA technology.
In theory, mRNA vaccines can trigger a stronger response from patients' immune system compared to the traditional flu vaccines.
To date, two companies are working on mRNA-based flu vaccines: Moderna and Sanofi.
Sanofi is collaborating with England-based vaccine developer Seqirus, with the two companies aiming for another mRNA flu test by early 2022. This will be in addition to the four flu vaccine candidates they have in the pipeline.
While the results for Sanofi’s flu vaccine efforts remain to be seen, experts are optimistic that it can drastically lower the number of deaths and severe cases annually.
For context, the most popular flu vaccine in 2018-19 flu season only had an efficacy rate of 29%.
Even with such low effectiveness, this vaccine prevents roughly 4.4 million flu cases in the United States alone. It also prevented 58,000 hospitalizations and 3,500 deaths.
Now, imagine how many lives a stronger candidate can save.
Although Sanofi appears to be late to the party in terms of its COVID-19 vaccine, the sheer efficacy of the company’s promising candidate with GSK makes it a powerful future contender as the leader of the pack.
Moreover, Sanofi is on the verge of resolving a pain point in the healthcare sector: the absence of a robust flu vaccine.
With its lineup of mRNA-based flu vaccine candidates, Sanofi is poised to discover potential game-changers in the industry and save millions of lives in the process.
Overall, Sanofi is a promising company sold at a reasonable price. More importantly, it prides itself on remarkable dividend history, paying and increasing dividends for 27 consecutive years.
Therefore, this dividend aristocrat is a good addition for investors on the lookout for quality and dependable stocks.