Biotechnology stocks that have fallen by over half since 2021 started are strewn around in the market.
While the share prices of COVID-19 stocks, such as Moderna (MRNA) and BioNTech (BNTX), have skyrocketed, the others in the sector are not as fortunate.
The biotechnology segment has been filled with relative horror stories from previous favorites like FibroGen (FGEN), Acadia Pharmaceuticals (ACAD), and Ultragenyx Pharmaceutical (RARE).
Among the companies hit with disappointing news, the gene therapy sector, which has stocks like bluebird bio (BLUE) and CRISPR Therapeutics (CRSP), appears to be having a tougher time than most.
So, where does this situation leave investors? Where should we look for value?
One tactic that would help value investors take advantage of this abysmal situation is studying Wall Street analysts' moves.
Look at target prices they set for the stocks they cover and then choose the companies trading the farthest below the expected price points.
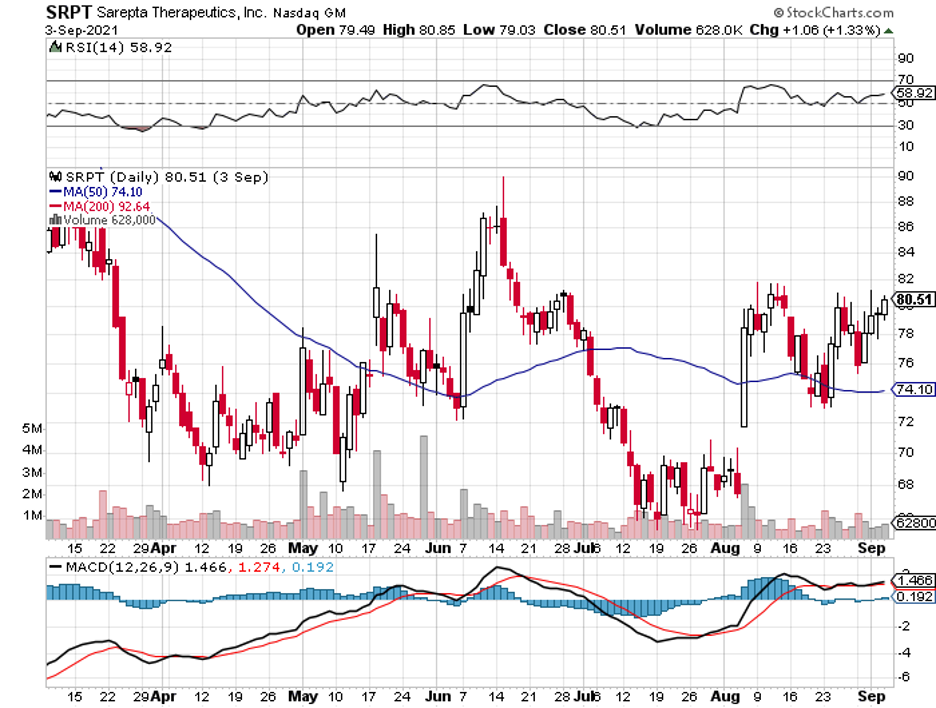
Among the biotechnology stocks struggling these days, one of the names that emerged as a truly promising investment is Sarepta Therapeutics (SRPT).
To say that Sarepta has been experiencing a disappointing year is an understatement. Shares of this company have gone on a 57% heart-stopping plunge since 2021 started.
In fact, this biotech’s nosedive is the third-worst in the midcap or larger stock sector based on the two key exchange-traded funds: iShares Biotechnology ETF (IBB) and SPDR S&P Biotech ETF (XBI).
Despite Sarepta’s abysmal performance, Wall Street still labels the stock a buy.
Sarepta’s decline started in early January when the trial results for its gene therapy for Duchenne muscular dystrophy (DMD) failed to impress investors.
However, the subsequent trial for the same gene therapy, dubbed as SRP-9001, is anticipated to yield better results.
Although the results are expected to be released by late 2022 or early 2023, holding the stock for now is estimated to deliver remarkable profits for patient investors.
At this point, Sarepta stock is trading at roughly $80 per share.
However, experts predict that the next results for SRP-9001 would push the shares to climb to over $200.
DMD, which is a rare degenerative condition characterized by gradual weakening of the muscles, affects about 16,840 people in the US.
So far, the US FDA has only approved a handful of treatments for DMD—three of which are RNA-based drugs developed by Sarepta.
Evidently, the company has a firm grasp of the condition and a proven track record of launching and marketing DMD-centered products.
More importantly, this makes Sarepta a dominant—if not the most dominant—force in the DMD market.
What makes their newest treatment, SRP-9001, different is that it works on DNA. Despite the extended timeline, Sarepta’s candidate remains the frontrunner in this endeavor.
Its closest threat is PF-06939926, an investigational drug from Pfizer (PFE) that uses the same technology as SRP-9001.
At the very least, this competitor is two years away from producing any tangible result.
By the time Pfizer reaches Phase 3, Sarepta’s SRP-9001 has already generated roughly $1 billion in sales.
Apart from the three approved drugs, Sarepta still has 34 drug development programs focused on two niches: RNA and AVV gene therapies. Among them, 7 are already in the clinical stage, including SRP-9001.
If successful, the company will reach $1 billion in revenues by 2023.
Another reason that makes Sarepta an attractive opportunity is its notably intact cash flow backbone—an achievement that’s worth pointing out considering its underwhelming SRP-9001 results.
The company also has an impressive history.
Sarepta has blossomed from its humble beginnings working as a contractor for the Department of Defense on the Ebola virus. Since then, it has developed its pipeline through acquisitions and launching effective treatments for rare diseases.
While Sarepta’s treatments are effective, they cannot cure DMD. They can only slow its progression.
This means continuous and life-long use among patients. For context, one treatment costs an average of $300,000 per patient annually.
Although it lost over 50% of its value, Sarepta still has enough capacity to rebound soon.
The factors that would help the company achieve this include the strong lineup of products generating solid and predictable revenues as well as its promising pipeline.
More importantly, its struggles with SRP-9001 don’t seem enough for the company to scrap the project altogether.
If anything, it proved that Sarepta can explore more potent treatments for DMD by looking into RNA technology—a path that the likes of Moderna and BioNTech have already found success in.
Overall, I think Sarepta still has a lot left in the tank. It operates in one of the most exciting sectors.
It’s worth bearing in mind that the biotech sector is ripe with innovation from companies with the ability to conjure up life-changing treatments—a value perceived as a crucial hallmark for massive gains.
Needless to say, Sarepta’s achievements in the DMD sector and its growth trajectory make it a shoo-in in this category.