Great investors have learned that the critical element when it comes to long-term investing is concentrating on stocks that hold a profound presence in their fields and that will continue to grow in the decades to come.
In terms of trends, the best thing to do is to determine something that will affect the world by generating millions—if not billions—of steady customers.
Among the stocks in the biotech industry today, one stands out to benefit from solid future demand for its products: Amgen (AMGN).
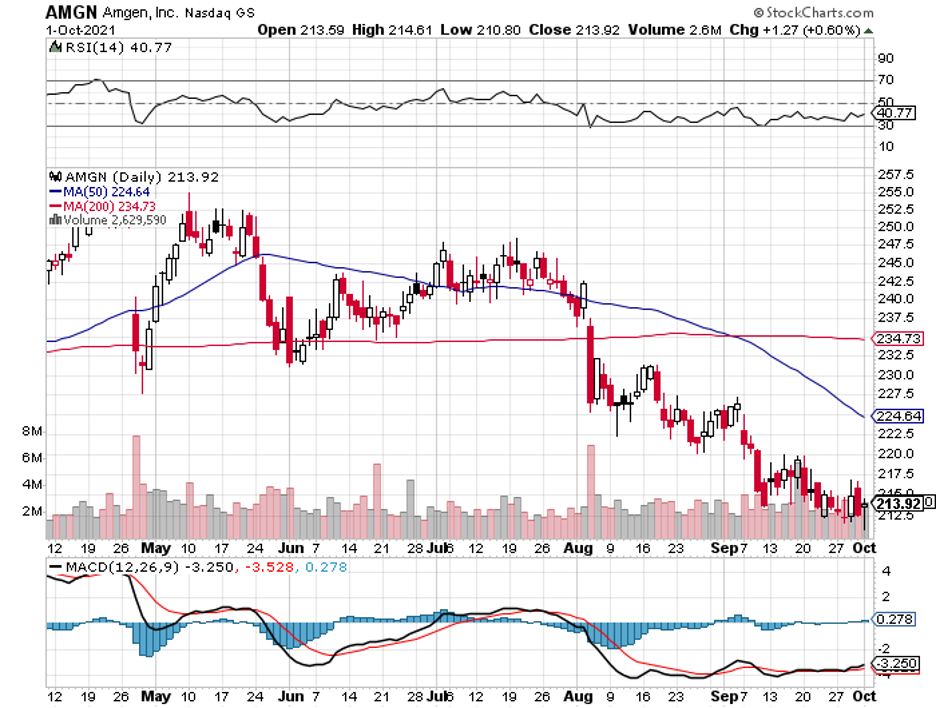
Amgen is one of the biggest biopharmaceutical companies across the globe, holding an equity market capitalization of roughly $127 billion. Despite its size, it simply can’t quite catch a break, with its share price continuing to slide in the past week.
While short-term investors may see this as a weakness, it’s moments like these that distinguish genuine value investors from the rest.
Let’s take a look at a company that has been thrown in the bargain bin for no apparent reason, and understand why this could be our opportunity.
A recent promising addition to Amgen’s pipeline is its experimental asthma drug, Tezepelumab, which it’s co-developing with AstraZeneca (AZN).
There are approximately 2.5 million patients worldwide who suffer from severe, uncontrolled asthma, accounting for almost 50% of all asthma-related expenses in the healthcare system.
This is because the majority of the 439,000 asthma-related hospitalizations, as well as 1.3 million emergency room visits annually in the US alone, are caused by severe, uncontrolled asthma.
Moreover, it was found that 1 in 5 severe asthma patients tend to develop a benign growth called nasal polyps in the sinuses of their noses. These can end up blocking their nasal passages, worsening their breathing problems, and diminishing their sense of smell.
This is the very market that Amgen’s Tezepelumab targets to help.
Tezepelumab is the first and only treatment that focuses on the symptoms of severe, uncontrolled asthma patients.
Considering the positive results of its late-stage trials, Amgen and AstraZeneca are confident that Tezepelumab will receive regulatory approval from the US FDA by the first quarter of 2022.
When that happens, this will mark Amgen’s first-ever foray into the asthma treatment sector—and it’s entering the market with a potential blockbuster to boot.
The global asthma market is projected to grow from $20.6 billion in 2020 to $37.3 billion in revenue by 2030.
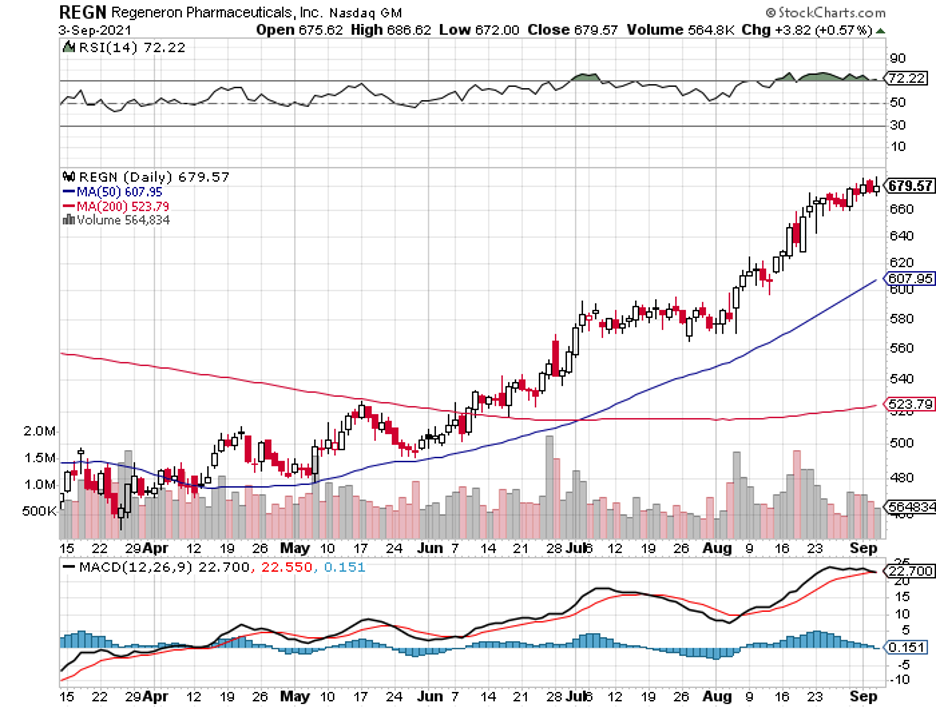
So far, the other names aiming to dominate this segment include GlaxoSmithKline (GSK), Regeneron (REGN), and Sanofi (SNY).
Considering the competition, a modest estimate is to expect Tezepelumab to seize at least 5% of the market share following its approval.
That would work out to roughly $1.9 billion in yearly revenue, divided between AstraZeneca and Amgen.
Taking into account that Amgen is forecasting its 2021 revenue to be within the range of $25.8 billion and $26.6 billion, the addition of $1 billion annually would surely move the needle.
Moreover, the cherry on top is that Tezepelumab is a clear indicator of the company’s efforts to diversify its revenue base and enter a market that it has yet to establish its presence.
Apart from Tezepelumab, Amgen has also been working on expanding its blockbuster lung cancer drug Lumakras, which generated $2.5 billion in annual sales.
To date, Lumakras is expected to emerge as a solid contender to unseat Merck’s (MRK) Keytruda in the lung cancer segment.
In addition, the company is studying how to utilize Lumakras as a potential treatment for colorectal cancer.
Amgen has also been expanding its pipeline of biosimilar candidates.
The most exciting candidates include its biologic version of Johnson & Johnson’s (JNJ) psoriatic arthritis and psoriasis medication Stelara, Regeneron’s chronic eye disease drug Eylea, and AstraZeneca’s rare disease treatment Soliris.
Even AbbVie’s (ABBV) impending loss of exclusivity for its top-selling rheumatoid arthritis drug Humira is under the company’s radar, with Amgen already prepared to launch its own biosimilar domestically in the form of Amjevita by 2023.
Getting the regulatory green light for these treatments would allow Amgen to poach on hundreds of millions, if not billions, in annual revenue from its competitors.
Apart from its pipeline candidates and strong performance in niche segments, Amgen has demonstrated a solid track record when it comes to capital returns via share buybacks.
In the second quarter of 2021 alone, the company has splurged on 6.5 million in shares repurchases. Amgen expects to reach a total of $3 billion to $5 billion in total repurchases throughout the year.
This strategy has pushed Amgen in its goal to continuously deliver market-beating returns in the past decade, as shown by its 451% total—overtaking the 384% return of the S&P 500.
Buying shares of a company when it’s declining can be an excellent step to set yourself up for future gains when the stock bounces back.
However, not all struggling stocks can recover.
So, it’s crucial to determine the reason for their fall. If the business itself is stable and solid, a decline in value might just be the opportunity you need to invest.
The truth is, nothing has actually changed when it comes to Amgen’s long-term stock growth prospects. It's still the company with a slew of top-selling products and more pipeline candidates expected to become blockbusters in the coming years.
All told, Amgen holds roughly 20 revenue-generating products in its diverse portfolio, and not a single drug accounts for over 20% of the company’s continuously rising top line.
Overall, I think Amgen is an A-rated company with a reasonable yield and a promising upside.