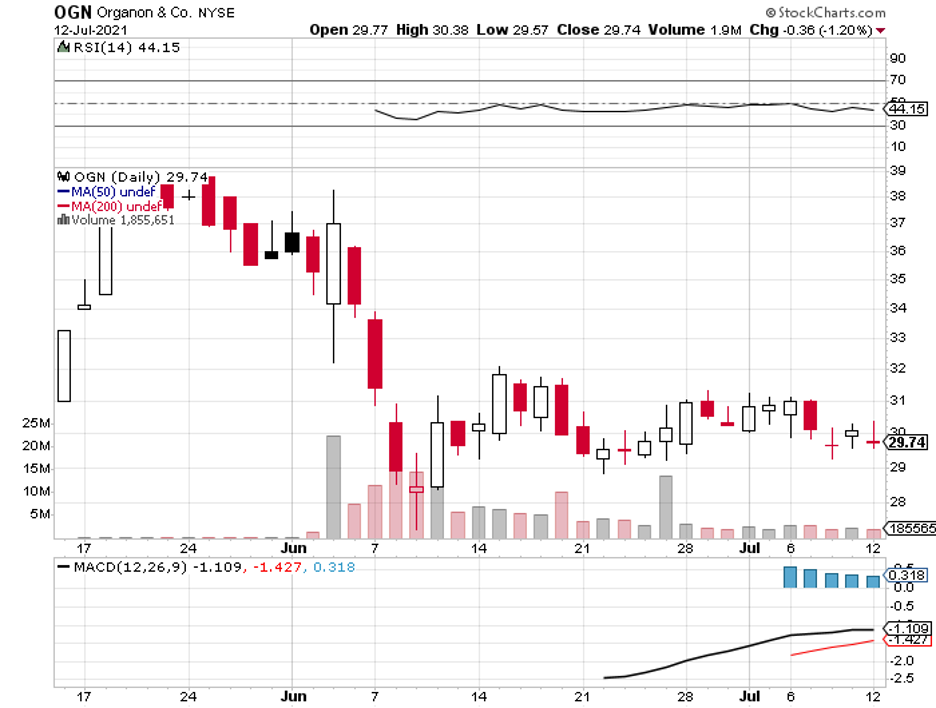
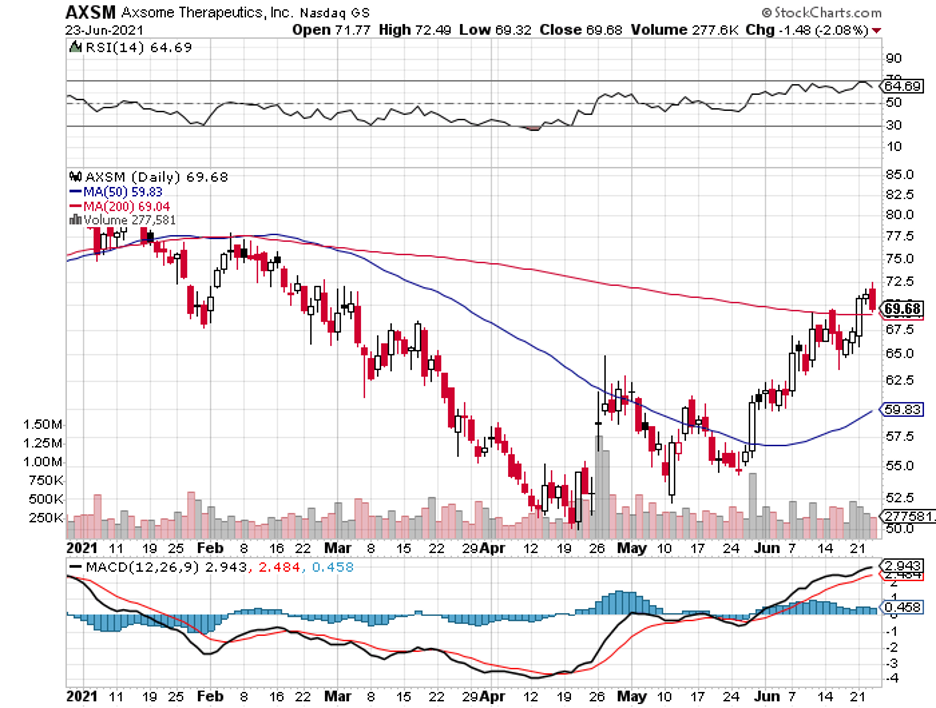
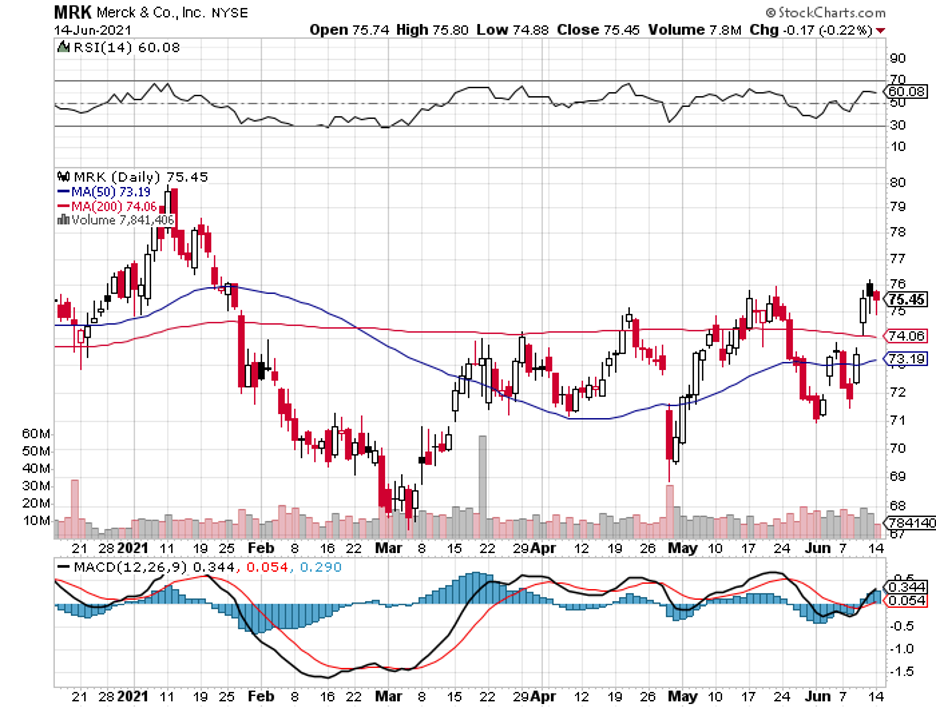
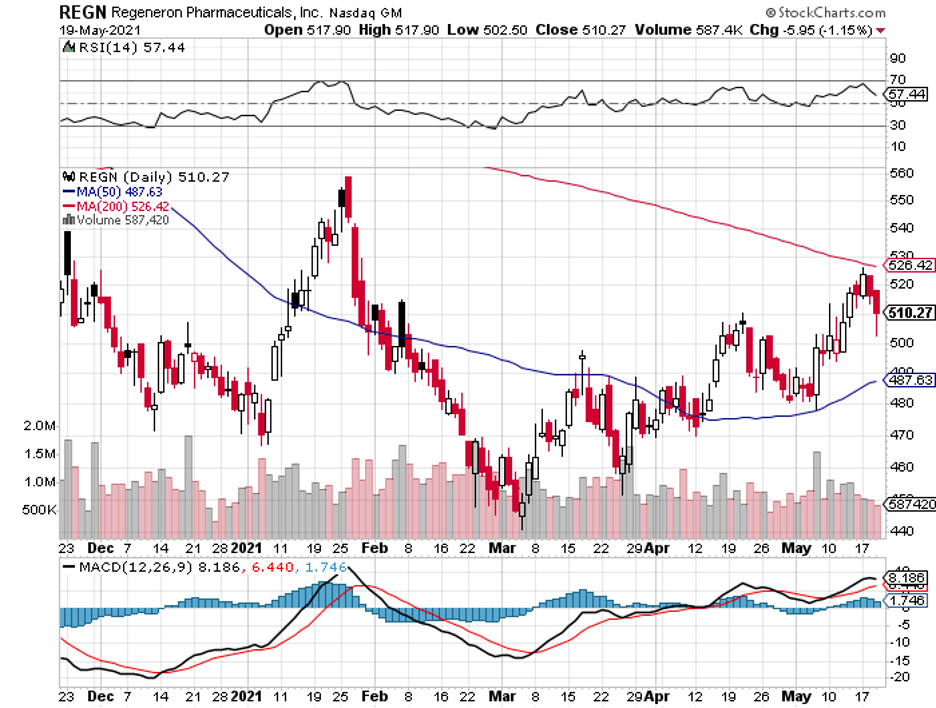
First, Alzheimer’s. Now, Parkinson’s.
Companies working on neurodegenerative diseases are on a roll.
After Biogen’s (BIIB) work with Aduhelm, another biopharmaceutical company has made notable progress: Bayer (BAYRY).
Merely six weeks after DA01 landed in the clinic, Bayer’s Parkinson’s disease drug candidate is getting into the fast lane.
This marks one of the major pipeline candidates that the German company picked up from its $1 billion acquisition of Versant Ventures in 2019.
DA01 is described as a “pluripotent stem cell-derived dopaminergic neuron therapy.”
In layman’s terms, Bayer collects donor cells that have the ability to develop into any other cell type in the body.
It will then engineer these versatile cells to turn into neurons that have the capacity to produce the neurotransmitter dopamine—aka the chemical your nervous system uses to transmit messages to nerve cells.
Those engineered neurons will then be transplanted into a part of the brain, called the putamen, which is in charge of our movements and learning.
What we know so far is that the next phase of the trial will determine the safety and tolerability of the cell transplantation a year following the procedure.
This will also tell us more about the cell survival rate after the transplant and the motor effects a year or two following the procedure.
Like Biogen’s Alzheimer’s candidate, the fast-track designation with the FDA could open doors for a speedy review or even an accelerated approval for Bayer’s DA01.
Aside from transplanting engineered cells into patients’ brains, the company is also looking into other options for Parkinson’s.
In October 2020, it shelled out $2 billion upfront to acquire Asklepios BioPharmaceutical or AskBio for its gene therapy research on Parkinson’s.
Roughly 1 million people in the US are suffering from Parkinson’s disease—a number that’s greater than the combined number of patients diagnosed with Lou Gehrig’s disease, multiple sclerosis, and muscular dystrophy.
What’s worse is that this is expected to climb to 1.2 million by 2030.
In terms of treatment cost, the combined expenses for Parkinson’s, including medical bills and lost income, are estimated to reach about $52 billion annually in the US alone.
The medications alone already amount to an average of $2,500 per year, with therapeutic surgery reaching up to $100,000 per person.
This is why it comes as no surprise that several companies have been working towards figuring out a more potent treatment or even cure for Parkinson’s.
One of the frontrunners is Prevail Therapeutics, a New York-based biotechnology company that’s focused on developing a gene therapy for this disease.
Following a successful Series B financing round in 2019, in which it secured $50 million in investments, the company eventually attracted the attention of big pharma.
By December 2020, it was acquired by Eli Lilly (LLY) for $880 million with the promise to help the smaller biotech company develop three of its most promising Parkinson’s candidates.
Another Parkinson’s-centered biotech company is Axovant Gene Therapies, which has been working on a single-dose treatment for neurodegenerative disease.
Its pipeline proved to be promising, as seen in its $74.7 million public offering just last February 2020, with the company maintaining its solid footing amid the pandemic.
By November, it rebranded itself as Sio Gene Therapies (SIOX).
Outside the US is Irish biotech firm Inflazome, which is working on a unique treatment for Parkinson’s.
Unlike the other candidates, the goal of Inflazome’s drug is to directly deliver the treatment to the affected neurons. That is, it plans to pass through the blood-brain barrier.
Its research attracted the Michael J. Fox Foundation, which granted it $1 million in funding, in March 2019.
Since then, the company’s progress has attracted the attention of other major biopharmaceutical companies with Roche (RHHBY), ultimately landing the acquisition in September 2020.
Of course, talks about neurodegenerative diseases wouldn’t be complete without Biogen.
On top of its Alzheimer’s work, the Massachusetts biotechnology giant has been collaborating with San Francisco-based Parkinson’s company Denali Therapeutics.
The two have been working on the development of three small molecular drugs for $560 million in upfront payments plus $465 million in equity investment into the smaller biotech.
In addition to these, we’re still waiting on what the rest of the major biopharmaceutical companies would come up with in the future.
Given that the likes of AbbVie (ABBV), Merck (MRK), Pfizer (PFE), and AstraZeneca (AZN) have all signed up publicly via the Critical Path for Parkinson's (CPP) consortium to tackle this debilitating disease, it’s safe to say that there’s hope for the future of this sector.
First, Alzheimer’s. Now, Parkinson’s.
Companies working on neurodegenerative diseases are on a roll.
After Biogen’s (BIIB) work with Aduhelm, another biopharmaceutical company has made notable progress: Bayer (BAYRY).
Merely six weeks after DA01 landed in the clinic, Bayer’s Parkinson’s disease drug candidate is getting into the fast lane.
This marks one of the major pipeline candidates that the German company picked up from its $1 billion acquisition of Versant Ventures in 2019.
DA01 is described as a “pluripotent stem cell-derived dopaminergic neuron therapy.”
In layman’s terms, Bayer collects donor cells that have the ability to develop into any other cell type in the body.
It will then engineer these versatile cells to turn into neurons that have the capacity to produce the neurotransmitter dopamine—aka the chemical your nervous system uses to transmit messages to nerve cells.
Those engineered neurons will then be transplanted into a part of the brain, called the putamen, which is in charge of our movements and learning.
What we know so far is that the next phase of the trial will determine the safety and tolerability of the cell transplantation a year following the procedure.
This will also tell us more about the cell survival rate after the transplant and the motor effects a year or two following the procedure.
Like Biogen’s Alzheimer’s candidate, the fast-track designation with the FDA could open doors for a speedy review or even an accelerated approval for Bayer’s DA01.
Aside from transplanting engineered cells into patients’ brains, the company is also looking into other options for Parkinson’s.
In October 2020, it shelled out $2 billion upfront to acquire Asklepios BioPharmaceutical or AskBio for its gene therapy research on Parkinson’s.
Roughly 1 million people in the US are suffering from Parkinson’s disease—a number that’s greater than the combined number of patients diagnosed with Lou Gehrig’s disease, multiple sclerosis, and muscular dystrophy.
What’s worse is that this is expected to climb to 1.2 million by 2030.
In terms of treatment cost, the combined expenses for Parkinson’s, including medical bills and lost income, are estimated to reach about $52 billion annually in the US alone.
The medications alone already amount to an average of $2,500 per year, with therapeutic surgery reaching up to $100,000 per person.
This is why it comes as no surprise that several companies have been working towards figuring out a more potent treatment or even cure for Parkinson’s.
One of the frontrunners is Prevail Therapeutics, a New York-based biotechnology company that’s focused on developing a gene therapy for this disease.
Following a successful Series B financing round in 2019, in which it secured $50 million in investments, the company eventually attracted the attention of big pharma.
By December 2020, it was acquired by Eli Lilly (LLY) for $880 million with the promise to help the smaller biotech company develop three of its most promising Parkinson’s candidates.
Another Parkinson’s-centered biotech company is Axovant Gene Therapies, which has been working on a single-dose treatment for neurodegenerative disease.
Its pipeline proved to be promising, as seen in its $74.7 million public offering just last February 2020, with the company maintaining its solid footing amid the pandemic.
By November, it rebranded itself as Sio Gene Therapies (SIOX).
Outside the US is Irish biotech firm Inflazome, which is working on a unique treatment for Parkinson’s.
Unlike the other candidates, the goal of Inflazome’s drug is to directly deliver the treatment to the affected neurons. That is, it plans to pass through the blood-brain barrier.
Its research attracted the Michael J. Fox Foundation, which granted it $1 million in funding, in March 2019.
Since then, the company’s progress has attracted the attention of other major biopharmaceutical companies with Roche (RHHBY), ultimately landing the acquisition in September 2020.
Of course, talks about neurodegenerative diseases wouldn’t be complete without Biogen.
On top of its Alzheimer’s work, the Massachusetts biotechnology giant has been collaborating with San Francisco-based Parkinson’s company Denali Therapeutics.
The two have been working on the development of three small molecular drugs for $560 million in upfront payments plus $465 million in equity investment into the smaller biotech.
In addition to these, we’re still waiting on what the rest of the major biopharmaceutical companies would come up with in the future.
Given that the likes of AbbVie (ABBV), Merck (MRK), Pfizer (PFE), and AstraZeneca (AZN) have all signed up publicly via the Critical Path for Parkinson's (CPP) consortium to tackle this debilitating disease, it’s safe to say that there’s hope for the future of this sector.