Financial markets have been incredibly volatile in the past months primarily due to the COVID-19 pandemic.
The situation was made even more unpredictable by the GameStop (GME) and bitcoin drama.
So it’s expected that investors are looking for guidance in this time of instability, and a good place to start is the Blackstone Group (BX).
Considering that the basic philosophy of this company is to “buy, fix, and sell,” it’s safe to say that Blackstone only puts its money, time, and effort in promising investments.
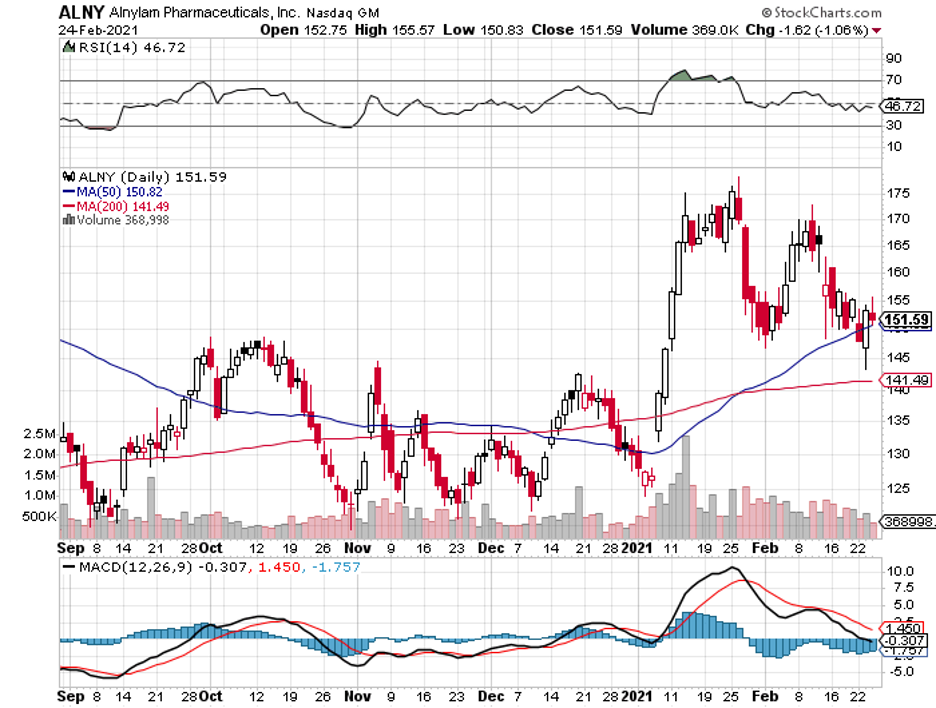
Around the time of the pandemic outbreak last year, Blackstone poured in roughly $2 billion investment in a biopharmaceutical company, Alnylam Pharmaceuticals (ALNY).
While Alnylam may be virtually unknown to the public, this is actually a promising company with an impressive backstory.
Founded in 2002, Alnylam is mainly known for its technology, RNAi or RNA interference.
This is a gene-silencing technique, which was discovered by Andrew Fire and Craig Mello back in 1998. The two won the Nobel Prize for it in 2006.
Even before the Nobel, Alnylam has already seen the potential of this technology and started developing it in the early 2000s.
For decades, this work had been underappreciated—up until the COVID-19 pandemic.
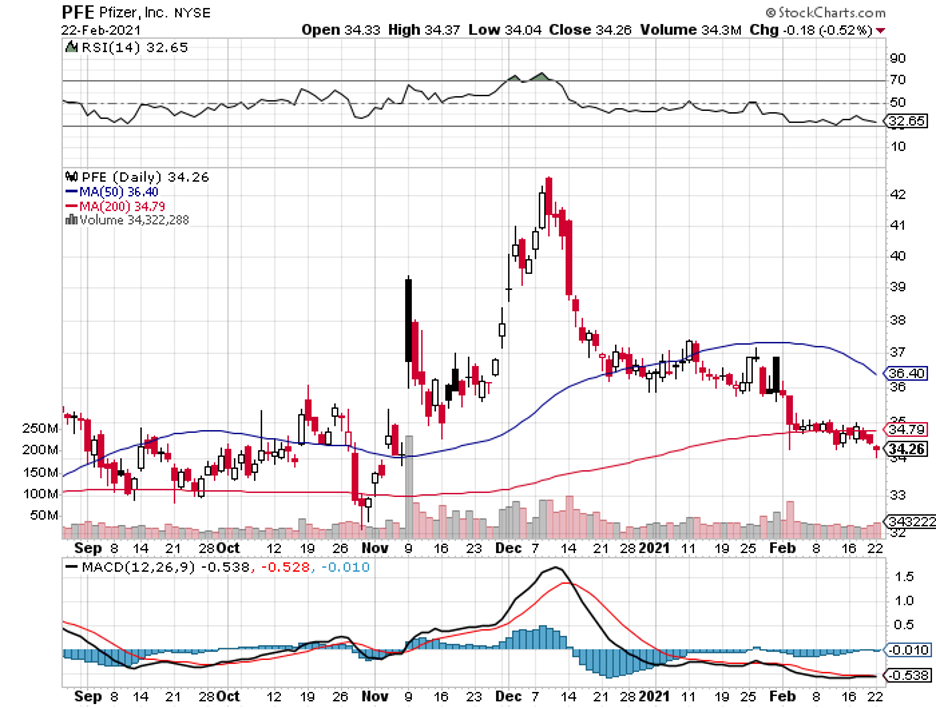
This is because the leading vaccine candidates right now, developed by Pfizer (PFE)-BioNTech (BNTX) and Moderna (MRNA), are both mRNA-based drugs.
Although the vaccine developers customized the technology, they still used the same delivery technique that Alnylam developed.
Clearly, there has been a lot of piggybacking on this discovery.
While Moderna, Pfizer, and BioNTech used the technology to create RNA-based drugs for the COVID-19 vaccines, Alnylam decided to utilize it to develop treatments for other diseases.
The first approval was hereditary transthyretin-mediated amyloidosis drug Onpattro, launched in 2018.
As of 2020, sales of this high-priced therapy reached roughly $300 million, ensuring that it was on pace with the company’s target.
Alnylam’s second approved treatment is ultra-rare genetic disease drug Givlaari, which hit the market early last year.
By the third quarter of 2020, sales of this acute hepatic porphyria drug climbed by $67 million despite the effects of the pandemic.
In the next decade, Givlaari is estimated to peak at $550 million annually.
By 2025, yearly sales for Givlaari and Onpattro are projected to hit roughly $1.5 billion in total.
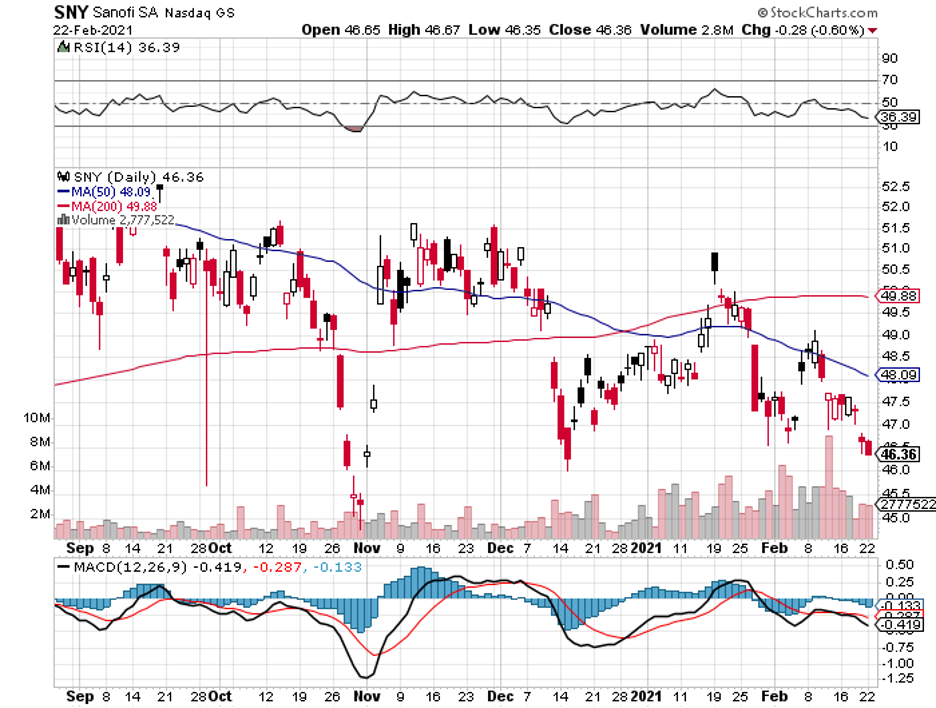
Riding this momentum, Alnylam has been collaborating with Sanofi (SNY) to develop another rare disease drug, Vutrisiran. This could rival the company’s own Onpattro.
Aside from Vutsiriran, Alnylam and Sanofi are also working on a potential novel hemophilia treatment, Fitusiran.
The latest treatment to gain approval is rare kidney disorder drug Oxlumo, which is estimated to net Alnylam roughly $380,000 per patient annually.
While this may be a hefty price tag, it’s expected that insurance companies and governments will be the ones to ultimately shell out the money for these rare disease drugs.
Before 2021 ends, Alnylam is expected to gain FDA approval for another potential blockbuster drug, Inclisiran. This is a cholesterol-fighting treatment, which is a work in progress with Novartis (NVS).
Over the past decade, Blackstone has been quietly stashing multi-billion-dollar stakes in the life sciences.
In 2020 alone, the company poured roughly $16 billion into the industry. This is its largest investment theme for the entire year.
While this business has yet to make a dent on Blackstone’s $600 billion assets, the attention that the companies have been getting is worth noting—and a good place to start is Alnylam.
For a better context of its potential, Blackstone invested $3 billion in a dating app called Bumble (BMBL) back in 2018.
Fast forward to 2021, this company is now worth approximately $14 billion following its recent IPO.
With a market capitalization of roughly $15 billion and for a company that’s not anticipated to generate over $1 billion in annual revenue until 2022, Alnylam’s current price might be considered high by some investors.
Looking at its pipeline though, which is filled with potential blockbusters, and its track record that shows that the company definitely knows how to launch new drugs to the market, I believe Alnylam stock is worth considering right now.